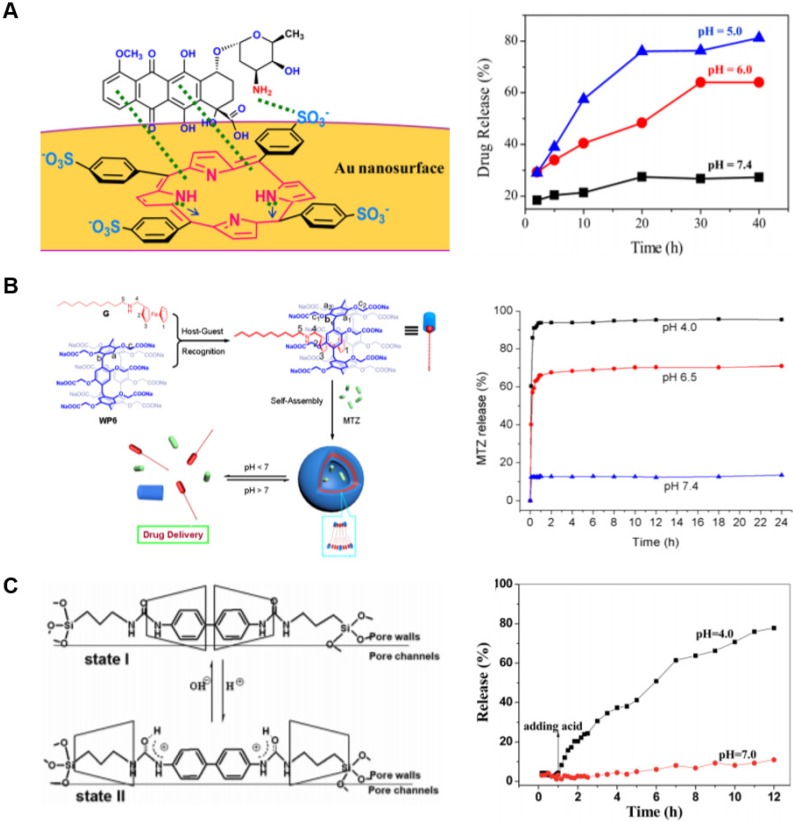
Figure 5.
Examples of drug release by pH change. (A) Porphyrin was used to noncovalently attach the anti-cancer drug doxorubicin to gold nanoparticles. The complex dissociates under acidic conditions as a result of competitive interactions with H+ ions. Figure adapted and used according to terms of use of 102. Copyright 2018 American Chemical Society. (B) Pillar[6]arene was used to form supramolecular amphiphiles with ferrocene-derived guests, which self-assembled into pH-responsive vesicles. Adapted with permission from reference 103. Copyright 2013 American Chemical Society. (C) A rotaxane system was designed as a mechanical gate across pores on the surface of porous silica. Acidic environments opened the gate by separating cyclodextrin macrocycles blocking the pore and allowing drug release. Adapted with permission from reference 104. Copyright 2016 Royal Society of Chemistry.