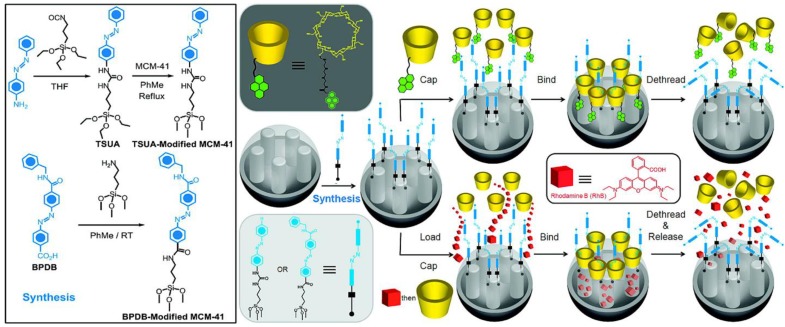
Figure 11.
Synthesis of 4-(3-triethoxysilylpropylureido)azobenzene (TSUA)- and (E)-4-((4-(benzylcarbamoyl)phenyl)diazenyl) benzoic acid (BPDB)-modified MCM-41. Two approaches to the operation and function of the azobenzene-modified MCM-41 NPs carrying nanovalves. Py-β-CD or β-CD threads onto the trans- azobenzene stalks to seal the nanopores. Upon irradiation (351 nm), the isomerization of trans-to-cis azobenzene units leads to the dissociation of Py-β-CD or β-CD rings from the stalks, thus opening the gates to the nanopores and releasing the cargo. Reprinted with permission from 45, copyright (2009) J. Am. Chem. Soc.