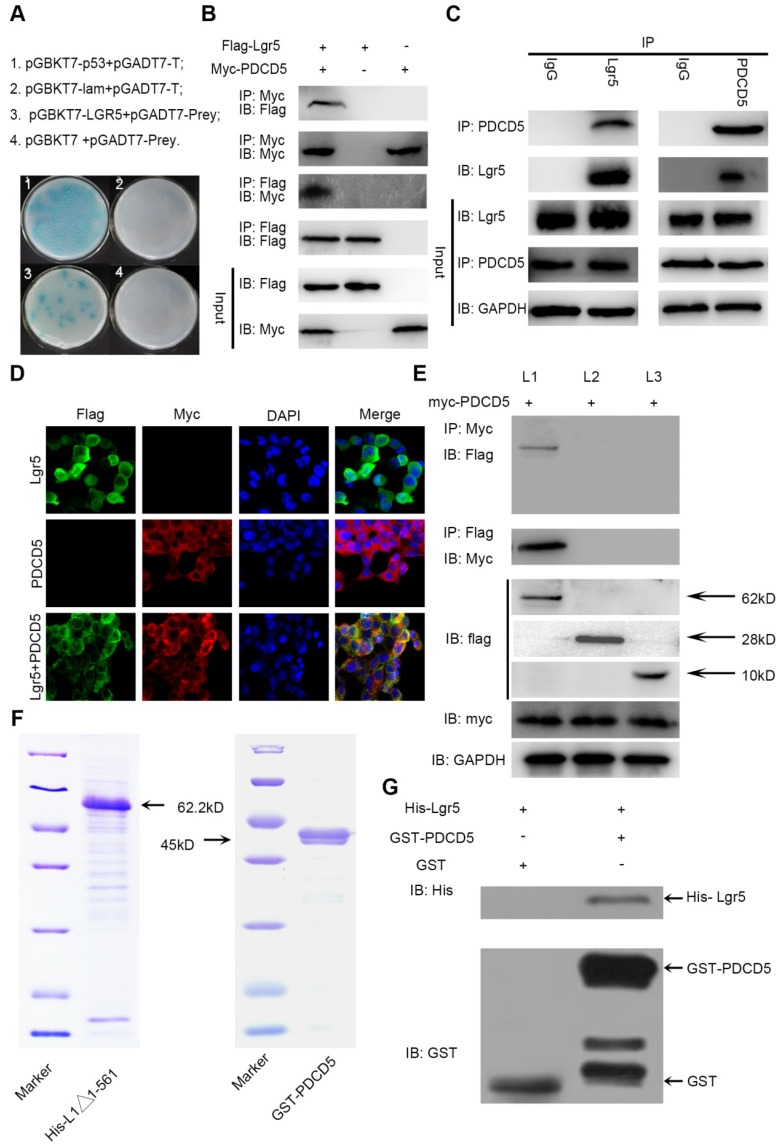
Figure 4.
The N-terminal extracellular domain of Lgr5 can directly bind to PDCD5. A. Yeast two-hybrid assay. Bait constructed with pGBKT7-Lgr5 was analyzed against prey constructed with human hepatocarcinoma tissue cDNA libraries. B. Co-IP of Lgr5 and PDCD5. Flag-Lgr5 and Myc-PDCD5 plasmids were transfected into HEK 293T cells. The precipitate was analyzed by western blotting with anti-Flag and anti-Myc antibodies. C. Interaction between endogenous Lgr5 and PDCD5 in cells. Cell lysates of HepG2 were prepared and used for Co-IP. The coimmunoprecipitates were analyzed by western blotting with anti-Lgr5 and anti-PDCD5 antibodies. D. Immunofluorescence was analyzed in HEK 293T cells transfected with Flag-Lgr5 and Myc-PDCD5 and detected with anti-Flag and anti-Myc antibodies. Fluorescence images were captured by confocal fluorescence microscopy. Scale bars, 20 µm. E. Flag-tagged full-length and deletion mutants (Lgr5, L1: Lgr5Δ22-561, L2: Lgr5Δ562-823, and L3: Lgr5Δ624-907) of the Lgr5 gene were cotransfected with Myc-PDCD5 in HEK 293T cells. Cell lysates were immunoprecipitated with an anti-Flag antibody, and the precipitates were analyzed with an anti-Myc antibody. F. His-Lgr5Δ22-561 and GST-PDCD5 proteins expressed in an E. coli system were detected by SDS-PAGE. G. Detection of His-Lgr5Δ22-561 bound to GST-PDCD5 or GST in a GST pull-down assay.