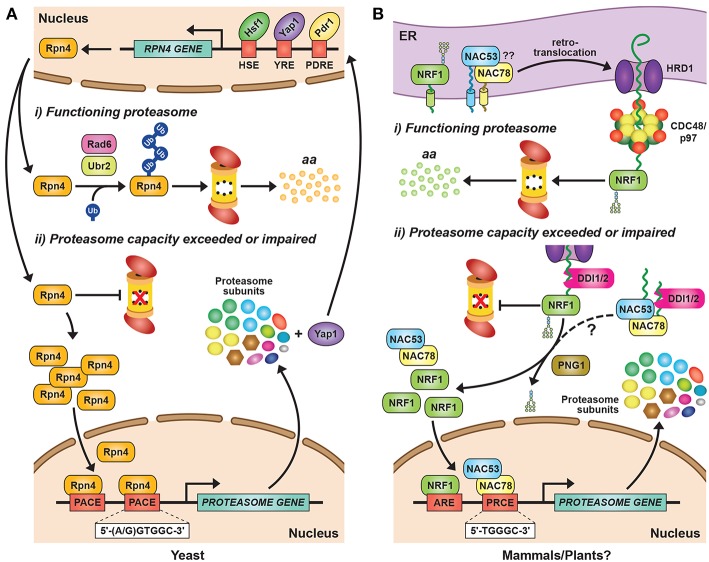
Figure 2.
Transcriptional Regulation of Proteasome Subunit Genes. (A) Regulation of proteasome gene expression in yeast. Expression of the transcription factor Rpn4 is controlled by various cis-regulatory elements bound by transcription factors, such as Hsf1, Pdr1, and Yap1. Rpn4 has an extremely short half-life and is continuously ubiquitylated and degraded by the 26S proteasome under normal growth conditions, a pathway initiated by the E2 Rad6 and the E3 Ubr2. A ubiquitin-independent route for Rpn4 degradation also exists. When proteasome capacity is exceeded or impaired, Rpn4 is stabilized and translocates to the nucleus, where it binds to a hexameric consensus nucleotide sequence [(A/G)GTGGC], known as the proteasome-associated control element (PACE), present in the promoters of most proteasome subunit genes. This binding leads to increased expression of proteasome subunits, along with additional genes involved in protein ubiquitylation, DNA repair, and other stress responses. One of the latter genes encodes Yap1, which can further increase Rpn4 levels via a positive-feedback loop. HSE, heat shock element; YRE, Yap response element; PDRE, pleiotropic drug response element; aa, amino acids. (B) Regulation of proteasome gene expression in mammals and plants. The mammalian transcription factor NRF1 is a type II endoplasmic reticulum (ER) membrane protein that is continuously retro-translocated to the cytosol via the ER-associated protein degradation (ERAD) pathway, a process requiring activity of the E3 ligase HRD1 and the AAA-ATPase CDC48/p97. Retro-translocated NRF1 is rapidly ubiquitylated and degraded by the 26S proteasome. When proteasome capacity is exceeded or impaired, NRF1 is stabilized during retro-translocation, where it is cleaved by the aspartyl protease DDI2. The resulting active form of NRF1 is deglycosylated by PNG1 and then translocates to the nucleus, where it binds antioxidant response elements (AREs) to activate transcription of its target genes, including those encoding proteasome subunits. The Arabidopsis transcription factors NAC53 and NAC78 control proteasome subunit gene expression and are predicted to be ER-localized transmembrane proteins. Given that Arabidopsis DDI1 also contains an aspartyl protease domain, we predict that transcriptional regulation of the proteasome in plants proceeds by a similar mechanism as in mammalian cells, by which the processed NAC53/78 dimer enters the nucleus and binds to proteasome-related cis-elements (PRCEs) to activate transcription.