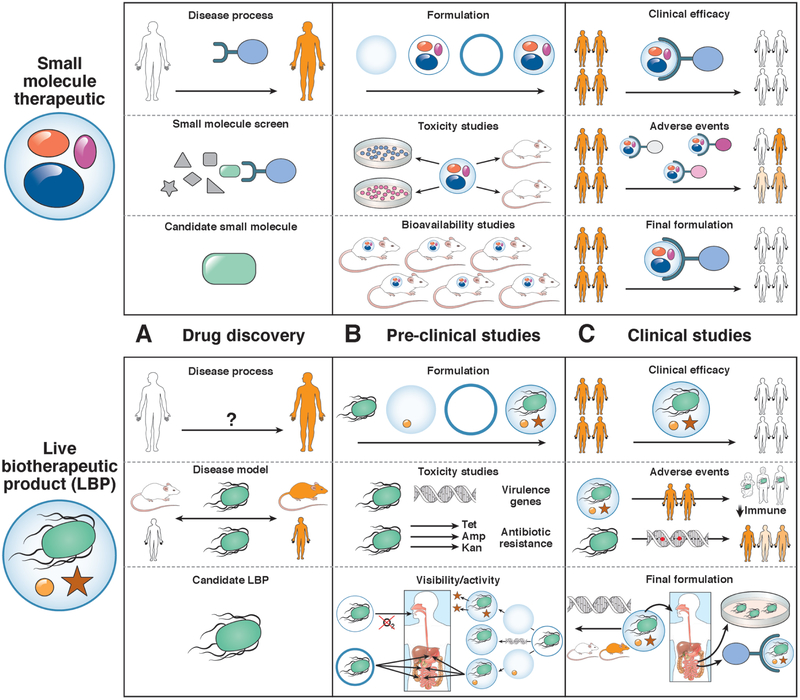
Figure 3. Challenges to Development of Therapeutic LBPs.
Compared with small molecules, LBPs have different challenges at each stage of drug development. Challenges are presented to drug discovery, preclinical studies, and clinical studies. A. In contrast to small molecules, which usually target a specific protein or class of feature of proteins, LBPs are identified based on their association with a disease phenotype in humans or mouse models. B. Small molecules require extensive toxicity studies, whereas LBPs are believed to be non-toxic but require assessments for virulence or antibiotic resistance. Preclinical studies of LBPs are not informative for bioavailability, but focus on viability or bioactivity, which can require specific encapsulation methods, adjuvants, or genetic modifications. C. Trials of LBPs require specific attention to adverse events related to transmission of the microbe, loss of its bioactivity, or off-target effects. Small molecules can also have off target effects, but these may be easier to predict, based on finding from preclinical studies. Early-phase studies of LPBs might be important for final formulation, because bioavailability and potential mechanisms can be assessed based on findings from small groups of patients.