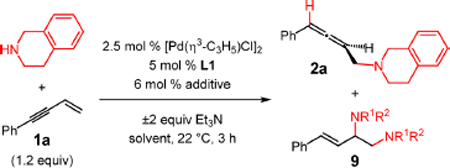
Table 5.
Initial Examination of Conditions for Enantioselective Enyne Hydroaminationa
 | ||||||
|---|---|---|---|---|---|---|
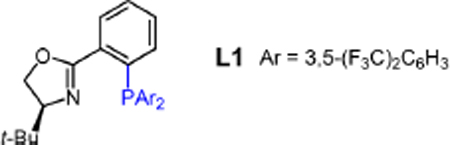 | ||||||
| entry | additive | Et3N (Y/N) | solvent | yield of 2a (%)b | 2a:9c | er of 2ad |
| 1 | NaBF4 | N | CH2Cl2 | <2 | – | – |
| 2e | NaBF4 | N | CH2Cl2 | 56 | 4:1 | 53.5:46.5 |
| 3 | NaBF4 | Y | CH2Cl2 | <2 | – | – |
| 4e | NaBF4 | Y | CH2Cl2 | 71 | 19:1 | 63:37 |
| 5e | NaBArF4 | N | Et2O | 73 | 13:1 | 69.5:30.5 |
| 6 | NaBArF4 | Y | Et2O | 48–73f | >20:1 | <52:48–74:26f |
| 7 | NaBArF4 | Y | CH2Cl2 | 65–70f | >20:1 | 54:46–82:18f |
See Table 1.
Isolated yield of 2a (single data point unless otherwise noted).
Determined by 400 MHz 1H NMR analysis of the unpurified mixture.
Determined by HPLC analysis (single data point unless otherwise noted).
Performed with isolated catalyst; see the Supporting Information.
Range for three experiments.
