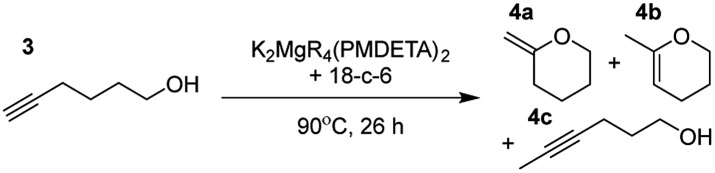
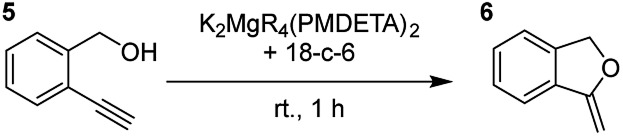
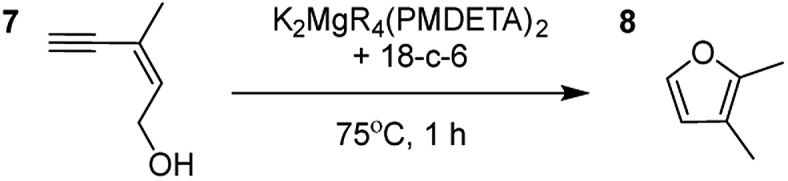
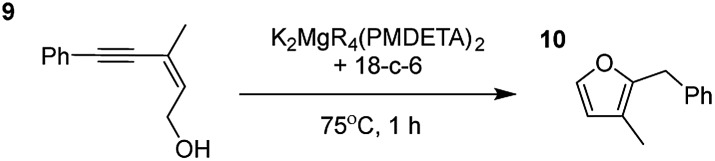
Table 2. Catalytic reactivity of K2MgR4(PMDETA)2 with the 18-crown-6 additive towards various structurally diverse alkynols.
aReactions were performed in a Young's cap NMR tube, using 0.6 mmol (1.2 eq.) substrate (3, 5, 7, or 9) and 0.025 mmol (5 mol%) pre-catalyst with 0.1 mmol (20 mol%/0.2 eq.) 1 consumed in converting the pre-catalyst to the ‘active catalyst’ (vide infra).
bCalculated from 1H NMR spectroscopic data by integration against an internal standard (10 mol% 1,2,3,4-tetraphenylnaphthalene).
cA stoichiometric quantity of crown ether co-catalyst used according to the alkali metal [i.e., 10 mol% 18-c-6 for 5 mol% K2MgR4(PMDETA)2].