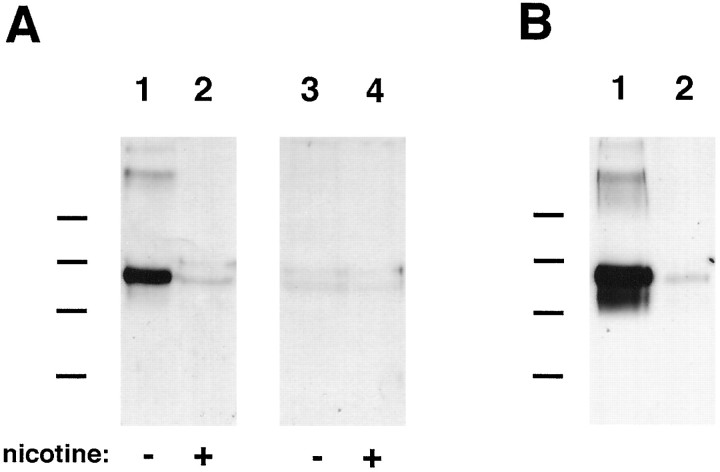
Fig. 6.
Immunoblot analysis showing α7 protein in PC12 AChRs. A, AChRs that bind αBgt were affinity-purified from PC12-B and -C cell extracts by adsorption to αBgt coupled to Actigel beads. The adsorbed material was eluted and analyzed by immunoblots probed with the anti-α7 mAb A7–1 and visualized using a horseradish peroxidase-coupled secondary antibody followed by enhanced chemiluminescence. A single species of about 60 kDa was obtained from PC12-C samples (lane 1). Nicotine at 250 μm (lane 2) blocked adsorption of the component to the αBgt-Actigel, indicating the specificity of the affinity purification. No components were obtained specifically when PC12-B cell extracts were analyzed by similar methods (lanes 3 and 4). Molecular weight standards (Bio-Rad) are as follows: phosphorylase B, 97 kDa; serum albumin, 66 kDa; ovalbumin, 45 kDa; and carbonic anhydrase, 31 kDa. Similar results were obtained in a second experiment using mAb A7–1 as probe and in two experiments using mAb 319 as probe. B, Total α7 protein from PC12-B and -C cell extracts was analyzed by immunoprecipitating protein with the α7-specific mAb 319 coupled to Actigel and probing immunoblots of the purified material with mAb A7–1. A species of 60 kDa component was obtained from both PC12-C (lane 1) and PC12-B (lane 2) cell extracts, although the latter contained much less of the component. Similar results were obtained in a second experiment. The band was absent when rat IgG-Actigel was substituted for the mAb 319-Actigel as a negative control (data not shown).