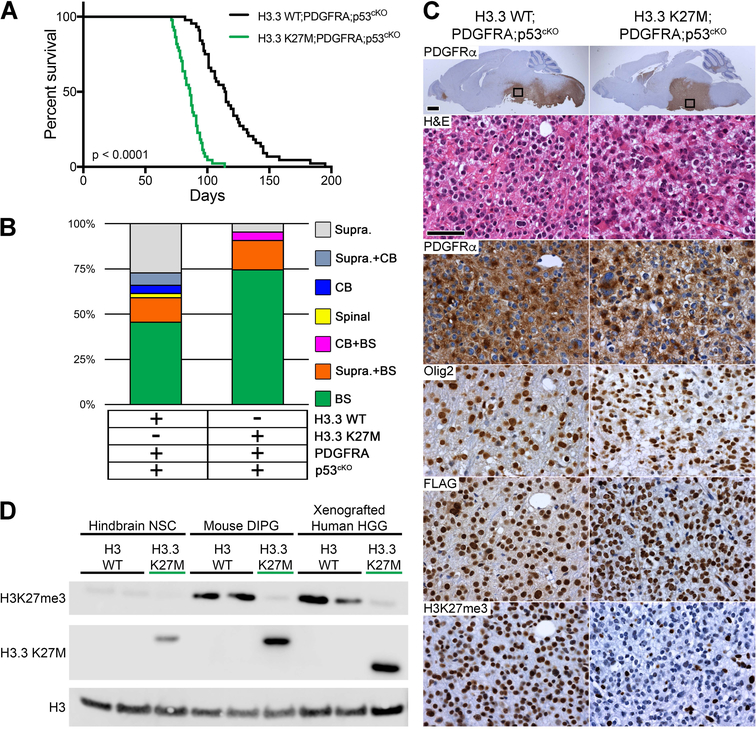
Figure 4. H3.3 K27M Accelerates DIPG Formation from Postnatal Neural Progenitors.
(A) Kaplan-Meier survival analysis of cohorts with induced PDGFRA;p53cKO combined with H3.3 WT (n = 44) or H3.3 K27M (n = 43; green), p < 0.0001. (B) Location of macroscopic brain tumors for cohorts shown in (A): Supra, Supratentorial; CB, Cerebellar; Spinal, Spinal cord; BS, Brainstem. (C) DIPG in H3.3 WT;PDGFRA;p53cKO and H3.3 K27M;PDGFRA;p53cKO mice. Sagittal sections immunostained with anti-human PDGFRα. Boxed areas in brainstem are shown at higher magnification for H&E, and IHC for PDGFRα, Olig2, FLAG-tagged H3.3, or H3K27me3 in representative HGG. Scale bar = 1 mm (whole brain images), 50 μm (higher magnification images). (D) Western blot of acid extracted mouse hindbrain NSCs, mouse DIPGs and xenografted human HGGs that express WT H3 (H3 WT) or the H3.3 K27M mutant from the endogenous H3f3a/H3F3A promoter. A H3.3 K27M-specific antibody is used to confirm mutation status. Epitope tagged mouse H3.3 K27M protein is slightly larger than human H3.3 K27M protein. Xenografted human HGG H3 WT is a cerebellar tumor and H3.3 K27M is a DIPG.