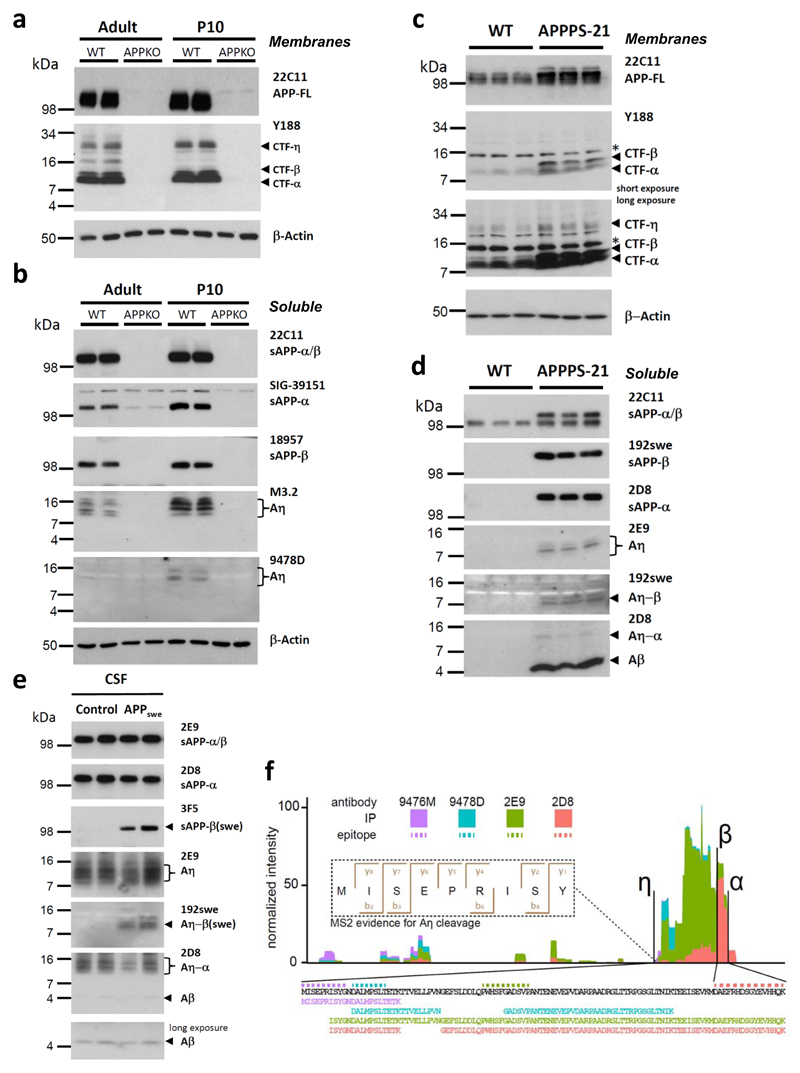
Figure 1. A novel proteolytic processing pathway of APP.
a, A 30 kDa N-terminally elongated APP-CTF-η fragment is detected in membrane fractions obtained from brains of adult (22 month) and postnatal day 10 (P10) mice using antibody Y188 directed against the C-terminus of APP. CTF-η is specifically found in young and old wild type (WT) mice but absent in APPKO19. In addition to this novel fragment, Y188 is detecting CTF-β and CTF-α. Full-length APP (APP-FL) was detected with antibody 22C11. β-Actin served as loading control. b, Aη was identified as several closely spaced peptides detected in the soluble fraction of adult and P10 mice by antibody M3.2. A similar pattern is detected by antibody 9478D that is specifically recognizing an N-terminal part of the Aη peptide (antibody 9478D may not be sensitive enough to detect the lower Aη levels in adult brain). sAPP-α and sAPP-β are shown as additional controls. APPKO brains were used as controls for antibody specificities. β-Actin served as a loading control. c, Higher levels of CTF-η are observed in RIPA lysates of APPPS1-21 mouse brains (long exposure) as compared to WT. Full-length APP (APP-FL) was detected with antibody 22C11. β-Actin served as a loading control. d, Soluble extracts of APPPS1-21 mouse brains contained Aη species detected by 2E9. Aη-β(swe) was selectively detected by antibody 192swe in addition to sAPP-β(swe). While 2D8 antibody detected robust levels of sAPP-α, only low levels of Aη-α could be detected in APPPS1-21 brain lysates due to the overexpression of APPswe transgene. e, Aη and Aβ were readily detectable in 10 μl of human CSF by antibody 2D8. Antibody 2E9 allowed the selective detection of Aη in the same samples, while 192swe specifically detected BACE1 cleaved Aη-β(swe) in the mutation carriers, but not in controls. f, Mass spectrometry analysis of peptides isolated by immunoprecipitation with antibodies 2E9, 2D8, 9478D and 9476M (supplementary Fig. S3 a and b). Peptide intensities were summed per amino acid residue and plotted in relation to each other. We detected peptides from the complete Aη-α sequence (see also supplementary Fig. S3c). The fragmentation spectrum of the N-terminal Aη peptide (APP505-5013) shows good coverage of the b- and y-ion series and an Andromeda score of 88.5 [Doi 10.1021/Pr101065j].