Abstract
Lung cancer is the leading cause of cancer-related death in the United States. Long non-coding RNAs (lncRNAs) are a class of regulatory molecules whose role in lung carcinogenesis is poorly understood. In this study, we profiled lncRNA expression in lung adenocarcinoma (LUAD) cell lines, compared their expression to that of purified alveolar epithelial type II cells (the purported cell of origin for LUAD), cross-referenced these with lncRNAs altered in primary human tumors, and interrogated for lncRNA whose expression correlated with patient survival. We identified LINC00261, a lncRNA with unknown function in LUAD, adjacent to the pioneering transcription factor FOXA2. Loss of LINC00261 was observed in multiple tumor types, including liver, breast, and gastric cancer. Reintroduction of LINC00261 into human LUAD cell lines inhibited cell migration and slowed proliferation by inducing a G2/M cell cycle arrest while upregulating DNA damage pathway genes and inducing phosphorylation-mediated activation of components of the DNA damage pathway. FOXA2 was able to induce LINC00261 expression, and the entire locus underwent hypermethylation in LUAD, leading to loss of expression. We have thus identified an epigenetically deregulated lncRNA, whose loss of expression in LUAD promotes the malignant phenotype and blocks activation of the DNA damage machinery, predisposing lung cells to cancer development.
Introduction
Lung cancer continues to be the leading cause of cancer-related death in the United States, with approximately 150,000 deaths reported annually (1, 2). Annual deaths attributed to lung cancer surpass colorectal, breast, and prostate cancers combined (3). Non-small cell lung cancer (NSCLC) represents the majority of new lung cancer cases, encompassing approximately 85% of all diagnosed lung cancers (4). Among NSCLC, the most commonly occurring subtype in the United States is lung adenocarcinoma (LUAD) (1). Understanding the specific drivers of LUAD can aid in developing targeted therapies, which has been effective for several other cancers (5, 6). Analysis of significant driver mutations in LUAD has identified KRAS, epidermal growth factor receptor (EGFR) and EML4-ALK translocations as the most prevalent mutations, present in over half of all cases (7). Targeted therapy directed towards EGFR mutations has shown a positive response to tyrosine kinase inhibitors, such as gefitinib and erlotinib (8). However, resistance soon arises, leading to overwhelming relapse rates (9–11). Furthermore, of LUAD cases screened, approximately 30% harbor no known oncogenic driver mutations (12, 13), emphasizing the need for a deeper understanding of the molecular mechanisms underlying carcinogenesis.
RNA plays many roles in cellular physiology, regulating processes integral to cellular survival. Long non-coding RNA (lncRNA) transcripts are a largely uncharacterized subtype of RNA defined as transcripts greater than 200 nucleotides in length with little to no protein coding capacity. Their high tissue specificity and temporal expression patterns suggest that they serve highly significant functions throughout development (14). In addition, lncRNAs exert a variety of functions throughout the cell, acting in cis locally, or in trans at different loci throughout the genome, playing critical roles in gene regulation and the development of cancer (15–17).
Large-scale profiling studies, such as that performed by The Cancer Genome Atlas (TCGA), have revealed thousands of differentially expressed lncRNAs in multiple cancers (12, 18), yet little is known of how these candidates function at a molecular level to regulate cellular phenotypes (19). Additionally, epigenetic alterations to lncRNA expression has been suggested to contribute to the cancer phenotype (20, 21). Only a few lncRNAs have been implicated in LUAD development (22–25), including H19, ANRIL, MEG3, NEAT1 and MALAT1 (25). The mechanisms of action for these lncRNAs are diverse, underscoring both the pivotal roles lncRNAs play in the development of cancer and our current limited understanding of lncRNAs full role in the pathogenesis of this disease.
We performed transcriptome-wide bioinformatic analysis coupled with molecular characterization of candidates to determine the functional relevance for lncRNAs with potential therapeutic applications in LUAD. LINC00261 was identified as being significantly downregulated in LUAD. Functional genomic studies demonstrated a fundamental role for LINC00261 in regulating G2/M cell cycle checkpoint arrest through activation of ATM phosphorylation. LINC00261 expression was lost in LUAD through epigenetic silencing of the LINC00261-FOXA2 transcription factor locus, and removal of DNA methylation silencing was able to reactivate LINC00261 expression. Our results identify LINC00261 as a novel tumor suppressor, whose epigenetic suppression results in worsening LUAD outcomes.
Materials & Methods
Cell Lines and Primary Cells
LUAD cell lines were obtained from the lab of Eric Haura or the American Tissue Culture Collection (ATCC, Manassas, VA) and fingerprinted to verify their identity prior to experimentation at the University of Arizona. Cells were verified mycoplasma-free every 2 months in the lab via established protocols (26). Remnant human transplant lungs were obtained in compliance with Institutional Review Board-approved protocols for the use of human source material in research (HS-07–00660) and processed within 3 days of death. Lungs were processed for primary alveolar epithelial type II cell isolation as in (27).
RNA- and whole genome bisulfite-sequencing (WGBS) and high dimensional analysis
For the cell lines and purified alveolar epithelial cells (AEC), 1 µg of RNA underwent RiboZero (Illumina, San Diego CA) depletion and subsequent library preparation using the Illumina TruSeq kit (Illumina, San Diego CA). Samples were multiplexed and underwent paired-end 50 bp sequencing on the Illumina HiSeq2000. FASTQ files were processed to remove bases 1–12 of all reads and low sequence complexity elements. Filtering was performed to retain only reads with Quality scores >20 for 90% of the read length. Once cleaned, FASTQ files were aligned to the Lincipedia2.1 transcriptome and RefSeqv58 (hg19) using Bowtie version 1.1. Transcripts were eliminated that had fewer than 10 reads across the dataset before differential analysis using EdgeR. For the transgenic H522-CMV-LINC00261 and H522-CMV-NEO controls, 1 µg of RNA underwent library preparation as above, then single end 76bp sequencing using the Illumina NextSeq 500 and analysis using Edge R. Raw FASTQ files were cleaned as above and aligned to the RefSeqv77 (hg38) transcriptome. Whole genome bisulfite sequencing (WGBS) processing and analysis were described previously (28).
PCR for cloning LINC00261 and promoter fragments
LINC00261 was synthesized in two segments using different methods due to high CpG and low complexity in the different fragments. The Phusion® High-Fidelity DNA Polymerase kit (New England Biolabs, Ipswitch, MA) was used for PCR amplification of the major exon of LINC00261. The single-stranded DNA oligonucleotide primers (Integrated DNA Technologies, Coralville, IA) used for PCR reactions are listed in Supplemental Table 1. Human genomic DNA (Promega, Madison, WI) was used as the template for all PCR reactions. The PCR reaction consisted of 1x Phusion HF buffer, 200 µM dNTPs, 0.5 µM forward primer, 0.5 µM reverse primer, 150 ng template genomic DNA from a human male (G147A, Promega), 3% DMSO, 0.3 µL Phusion DNA polymerase, and water to a total volume of 30 µL. LINC00261 upstream exons 1–3 were synthesized by IDT-DNA (Coralville, IA) using their gene synthesis technology and cloned along with the major exon into the pCMV6-vector backbone (PS100001, Origene).
Generation of stable cell lines expressing LINC00261
Mycoplasma-negative A549 cells underwent SNP-typing for cell-line verification and were then transfected with either the linearized LINC00261 shRNA construct or scrambled control shRNA (HC137604, Origene). 70,000 cells per well were plated into a 24-well dish and puromycin (0.625 µg/mL) was used for selection. H522 LUAD cells were transfected with either the linearized full-length LINC00261 plasmid or the control C-terminal Myc-DDK tagged pCMV-6 entry vector. Optimized transfection conditions for H522 cells consisted of 80,000 cells per well with a G418 concentration of 166 µg/mL. Stable cell lines were assayed by qRT-PCR every third passage to verify stable knockdown was maintained.
Data Access
All datasets are deposited in the public GEO database (GSE110025).
RNA Isolation and Quantitative Real Time-PCR (qRT-PCR)
RNA was harvested from cells using TRIzol reagent according to the manufacturer’s protocol (Sigma, St. Louis, MO). cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-rad, Hercules, CA) according to the manufacturer’s protocol.
Proliferation Assay
Cell proliferation was measured by seeding 10,000 cells per well in sets of 4 wells in a 24-well dish. One well of each set was trypsinized at 24-hour intervals over a period of 4 days and counted using a Bright-Line hemocytometer (Sigma-Aldrich, St. Louis, MO). Three technical replicates were performed on three different stably transfected cell lines per assay.
Transwell Migration and Invasion Assay
Transwell migration and invasion assays were performed as previously described (29). Briefly, cell migration was measured using Corning Transwell inserts (8-μm pore size, Corning, Union City, CA). In each well, 50,000 cells were added to the upper chamber, suspended in 100 μL of serum-free medium (RPMI 1470, 1% penicillin/streptomycin, 0.1% bovine serum albumin) (RMBIO, Missula, MT). In the bottom chamber, 600 μL of complete medium (RPMI 1470, 1% penicillin/streptomycin, 10% fetal bovine serum (FBS)) was added. After 24-hour incubation, the top of the membrane was dried with a cotton-swab to remove any remaining non-migrated cells. Transwell inserts were fixed with 70% ethanol for 10 minutes, dried, and stained with 0.2% crystal violet (Santa Cruz Biotechnology, Dallas, TX). Using an inverted microscope with a magnification of 100x, migrated cells were counted in three randomly selected fields and averaged. Cell invasion was measured by coating wells with 15 μg of Matrigel (Corning, Union City, CA, USA) diluted in 0.01M Tris (pH 8.0) and 0.7% NaCl (Promega, Madison, WI; Amresco, Solon, OH) and allowing them to dry for 4 hours in 37° C prior to adding cells. Three biological and technical replicate experiments were performed for each assay.
Soft Agar Assay
Colony formation was measured by suspending 2,000 cells in soft-agar growth medium (0.3% agar, 1x RPMI 1470, 1% penicillin/streptomycin, 10% fetal bovine serum) in 6 well plates pre-coated with a solidified 0.5% agar/culture medium base. Twice a week, 100 µL of culture medium was added to the top of the agar layer. After three weeks, cells were stained with 6.0% glutaraldehyde and 0.1% crystal violet and imaged using an Olympus IX51 Inverted System microscope (40x magnification) with Olympus Qcolor3 camera. Quantification was performed by counting colonies from three random fields using QCapture Software (Surrey, BC, Canada) in three biological replicates. A colony is defined as area >10,000µm2. Three technical replicates were performed for each assay.
Flow Cytometry
Cells were plated onto Corning six-well tissue culture dishes (Corning, NY). Once 70% confluent, cells were hypotonically lysed in 300 µl of DNA staining solution consisting of 0.5 mg/mL propidium iodide (PI), 0.1% sodium citrate, 0.05% Triton X-100 (Sigma-Aldrich). Lysates were filtered using a 40 µm nylon cell strainer (BD Falcon, Franklin Lakes, NJ) to remove cell membranes and debris. PI-stained nuclei were detected using a PL-2 detector with a 575 nm band pass filter on a Beckman-Coulter (Fullerton, CA) fluorescence-activated cell sorter analyzer with laser output adjusted to 488 nm. 10,000 nuclei from the total population were analyzed per sample at a rate of 100–200 nuclei/s. The percentages of cells within the G1, S and G2/M phases of the cell cycle were determined by analyzing the output histogram using FlowJo v10.1 (San Diego, CA). Technical triplicates were averaged, and statistics performed on three biological replicates.
Scratch Assay
400,000 cells per well were plated into a 6-well dish and the cells were grown to confluence. The plate was then vertically scratched using a P20 pipette tip, and visually inspected using an inverted phase contrast microscope. After 24 hours, the cells were washed with phosphate-buffered saline (PBS) and media were replaced, followed by visualization. The plates were then analyzed with T-Scratch software (30). The protocol was modified from (31). Technical quadruplicates were averaged, and statistics performed on three biological replicates.
Tumor xenografts
All mouse experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of USC, protocol # 20633. Eight-week-old female athymic nude mice were purchased from Jackson’s Laboratory (Jackson Laboratory, Bar Harbor, ME). All animal studies were performed in compliance with the University of Southern California Institutional Animal Care and Use Committee (IACUC) guidelines. H522 CMV-NEO and CMV-LINC00261 cells are were suspended in 150μl PBS with 50% Matrigel and subcutaneously injected in the dorsal flanks of the mice (1×106 cells per flank). Tumors were measured 3 times per week and their volumes (V) are calculated by the previously published formula V = lw2/2, where l and w are the larger and smaller length diameters, respectively (32). Mice were euthanized after 6 weeks by intraperitoneal injection of Euthasol at the experiment end point and tumors are excised and weighed.
Western Analysis
Total protein lysates were obtained from both H522-CMV-LINC00261 cells and H522-CMV-Neo controls using radioimmunoprecipitation assay (RIPA) buffer containing 1 mM phenylmethylsulfonyl fluoride (PMSF). Protein lysates were run on 10% Tris polyacrylamide gels and then electrophoretically transferred to Immuno-Blot PVDF membranes (22 volts overnight transfer). The membranes were then incubated overnight at 4°C with antibodies from the Cell Signaling DNA damage repair kit (CS#9947), as well as beta-actin (Cell Signaling #4970), BRCA2 (Origene #TA313520), and phospho-BRCA2 (Invitrogen #PA537499). Membranes were blocked for 1 hour in 5% non-fat dry milk (NFDM) at room temperature and horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin was applied at a dilution of 1:10,000 for 2 hours at room temperature. HSP90 (GTX109753, GeneTex, 1:1000) was used as loading control and the blots were visualized using a Molecular Image ® ChemiDoc ™XRS+ (BioRad, Hercules, CA). Analysis software used was ImageJ (NIH). Ku-55933 (Sigma-Aldrich, St. Louis MO) was used as a specific inhibitor of ATM kinase phosphorylation. Representative blots of three independent experiments shown.
Luciferase Reporter Assay
Constructs were transfected into A549 cells using FugeneHD (Promega, Madison, WI) and H522 cells using Lipofectamine2000 (Thermo Fisher, Waltham MA). Luciferase assays were conducted as previously described (28). Technical triplicates were averaged, and statistics performed on three biological replicates.
Statistical Analysis
Statistics for biological experiments were calculated using Prism5. Two-sided paired t-tests were used for cell line comparisons between test and controls. ANOVA was used for trend tests. P values are denoted as follows: “*” ≤ 0.05, “**” ≤ 0.01, “***” ≤ 0.001.
Results
Identification of LINC0261 as a tumor suppressor in LUAD
To identify LUAD-specific candidate lncRNAs, we analyzed 16 different LUAD cell lines for their whole lncRNA transcriptome as defined by lincipedia2.1 (Figure S1A). This revealed ~32,000 lncRNAs expressed to some level in LUAD. Differentially expressed lncRNAs between cancerous cells and normal human alveolar epithelial cells (AEC), the purported cells of origin for LUAD were identified by comparing LUAD cell lines to previously generated whole transcriptomic profiling of purified AEC from three human donor lungs not used for transplant (27). Comparison of lncRNA expression revealed that 833 lncRNA transcripts (649 genes) were differentially expressed between LUAD cell lines and AEC (Figure S1B, Supplemental Table 2). To exclude lncRNAs differentially expressed due to effects of in vitro cell culture, we compared this set of lncRNAs to those differentially expressed in publicly available datasets of primary human LUAD tumors profiled by TCGA, as previously determined by the Mather group (33), resulting in a narrowed list of 16 lncRNAs with potential relevance in both primary human tumors and cell line model systems.
While previous transcriptomic analyses have determined that thousands of genes are differentially expressed in cancers when compared to adjacent normal tissues, few are ‘drivers’ of carcinogenesis. The majority of alterations are ‘passive’ and the result of tumorigenesis, not causal. To assess which of these lncRNAs may be driving carcinogenesis, we evaluated survival outcomes and stage at loss of expression. Six of the candidate 16 lncRNAs had significant effects on survival, and LINC00261 emerged as the top candidate with a dramatic stage-dependent effect on expression (Figure 1A) and survival (Figure 1B). Using qRT-PCR, LINC00261 was significantly downregulated in the panel of LUAD cell lines compared to the purified primary AEC (Figure 1C). To determine whether loss of expression was LUAD-specific or a more generalized phenomenon across cancer types, we extracted the expression of LINC00261 in many TCGA-profiled cancer types using lncRNAtor (34). LINC00261 loss is observed in multiple epithelial cancers (Figure 1D), with effects on survival and stage-dependent expression in liver and breast (Figure S2A, B), as well as previous reports in gastric cancer (35), indicating that LINC00261 is a candidate of high interest as a potential tumor suppressor across multiple epithelial cancer types. In addition, LINC00261 expression was significantly correlated with tobacco smoking history in LUAD, suggesting an environmental trigger may initiate loss of this gene in the development of cancer (Figure S2C).
Figure 1.
Loss of LINC00261 expression is correlated with decreased survival in lung adenocarcinoma. A. Stratification of LINC00261 expression of 515 LUAD samples from TCGA (12) dataset by stage. “Adj. NTL” = Adjacent non-tumor lung. ANOVA tested all groups, difference is significant between normal and stages 1, 2, and 3. Stage 4 = ns (TANRIC). (45). B. Overall survival of patients stratified by expression level of LINC00261 derived from KMplot (46). Red = high LINC00261 expression, Black = low LINC00261 expression. C. qRT-PCR of LINC00261 expression in LUAD cell lines and primary AEC. D. Stratification of LINC00261 expression in multiple TCGA (12) datasets (lncRNAtor) (34). See TCGA website for acronym definitions https://cancergenome.nih.gov.
LINC00261 expression reduces proliferation, migration and initiates G2/M cell cycle arrest in LUAD cells
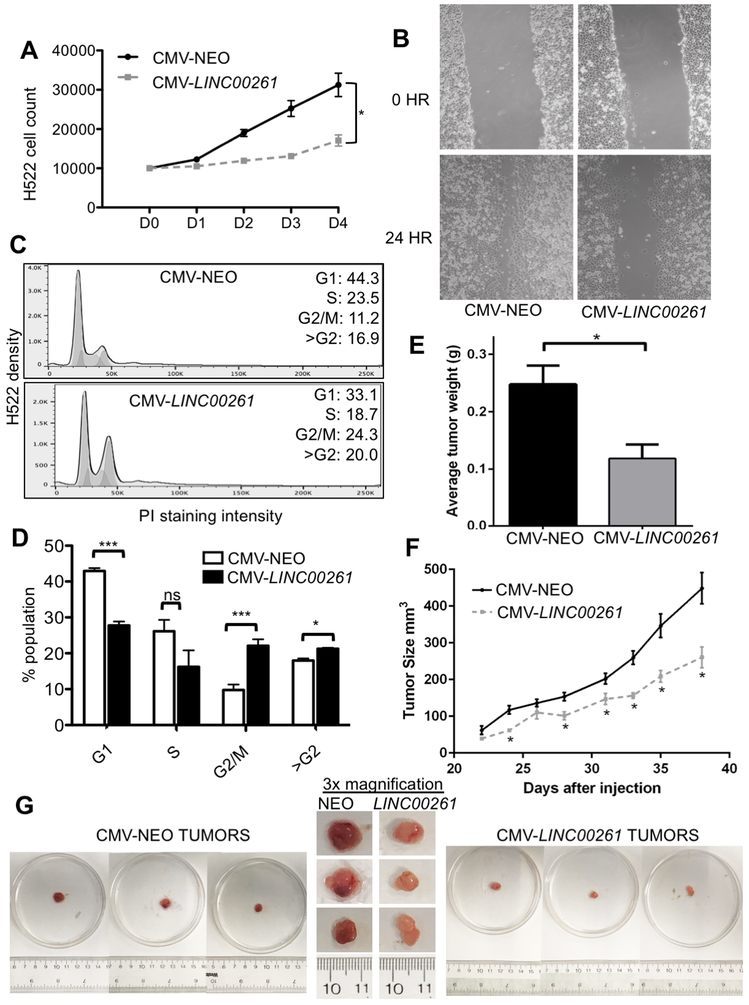
To determine if LINC00261 plays a functional role in LUAD carcinogenesis, we constructed an ectopic expression vector containing a CMV promoter and the full-length LINC00261 transcript. H522 LUAD cell lines were chosen for ectopic reintroduction as they lack endogenous LINC00261 expression (Figure S3A). Stable reintroduction of the CMV construct resulted in a level of expression equivalent to endogenous levels of LINC00261 expression in primary AEC (Figure S3B). Ectopic expression of LINC00261 resulted in a significant decrease in proliferation of H522 cells (p<0.01, Figure 2A) compared to CMV-NEO vector control over the course of four days. In addition to increased proliferation, one of the hallmarks of cancer is acquisition of migratory capacity, leading to metastasis. The migratory capability of H522 CMV-LINC00261 stable cell lines was tested through a scratch assay. LINC00261 was able to decrease migration of H522 LUAD cells compared to NEO controls (Figure 2B). Quantification over the course of four independent experiments using TScratch revealed that LINC00261 significantly blocks migratory capacity in vitro (Figure S3C).
Figure 2.
Ectopic expression of LINC00261 blocks proliferation and inhibits tumorigenesis through induction of G2/M cell cycle arrest. A. Quantification of cell proliferation of H522-CMV-LINC00261 and H522-CMV-NEO controls using trypan blue staining. B. Scratch assay of H522-CMV-LINC00261 cells compared to CMV-NEO vector control. Performed in 3 separate stable cell lines and technical quadruplicates (N=4). Representative image shown. C. Propidium Iodide (PI) staining and FACS analysis of CMV-LINC00261 and CMV-NEO controls. Population was analyzed using FlowJo v10.1. N=3 (≥10,000 cells per sample). Representative FACS analysis shown. D. Quantification of FACS analysis in (C). E. Average weight of H522 xenograft tumors after excision. H522-CMV-NEO = 8 tumors, H522-CMV-LINC00261 = 5 tumors. F. Size of xenograft tumors as measured over time. Significance was determined by a paired T-test between test conditions at each time point as shown. (*) = p<0.05. G. Images of tumors of H522 CMV-NEO and H522 CMV-LINC00261 origin. Original images of tumors from CMV-NEO controls are shown on the left, tumors from CMV-LINC00261 on the right. Center panel consists of 3x magnifications alongside ruler for size comparison.
To determine how LINC00261 blocks cellular proliferation, we performed cell cycle analysis on H522-CMV-LINC00261 and H522-CMV-NEO controls. FACS analysis demonstrated that reintroduction of LINC00261 arrests H522 cells in the G2/M phase of the cell cycle (Figures 2C, S3D). Quantification revealed a significant shift in the population from the G0/G1 to the G2/M phase of the cell cycle (Figure 2D). Finally, a key hallmark of defining a gene as a tumor suppressor is the ability in vivo to inhibit tumor formation. H522-CMV-LINC00261 cells alongside CMV-NEO controls were implanted into nude mice and the growth of the tumors measured for 6 weeks. LINC00261 resulted in decreased tumor weight as compared to NEO controls (Figure 2E). LINC00261 was also able to inhibit tumor growth over time (Figure 2F). The LINC00261-expressing tumors also showed a decrease in vascularization relative to empty vector controls (Figure 2G).
While the majority of LUAD primary tumors and LUAD cell lines lack expression of LINC00261, there are a few stage 4 primary tumors and aneuploid cell lines that express measurable endogenous LINC00261, including the A549 LUAD cell line (Figure 1C). To determine if ablation of LINC00261 was able to affect the hallmarks of cancer phenotypes, we generated stable knockdown of LINC00261 using short hairpin RNAs (A549-shLINC00261) in A549 cells, one of the cell lines that still express endogenous LINC00261 (Figure 3A). Ablation of LINC00261 in A549 cells caused a significant increase in A549 cell proliferation (Figure 3B), and also significantly increased colony formation (Figure 3C, D) and invasion (Figure 3E, F). Migration of A549 cells was also affected by knockdown of LINC00261; however, this result did not reach statistical significance (Figure S4A). FACS analysis of A549-shLINC00261 showed a significant increase in the proportion of cells with >G2 DNA content (Figure 3G, H), suggesting that lack of LINC00261 results in chromosomal instability and aneuploidy. Consistent with this, we began to observe an increase in cells with higher order DNA content as evidenced by the increase in macronucleated cells (Figure S4B). These cells accounted for ~10–15% of the population at any given time, similar to our FACS findings.
Figure 3.
Knock down of LINC00261 results in increased proliferation and migration in lung cell lines. A. Generation of stable A549 LUAD cell lines with short hairpin knockdown of LINC00261. Stable cell lines (n=3) were generated and verified by qRT-PCR. B. Cell proliferation was counted using trypan blue staining. Statistical analysis was performed on doubling times between stable cell lines shown (N=4). C. Representative field (40x magnification) showing colony formation assay of A549-shScrambled and A549-shLINC00261 cells after 3 weeks of growth in 0.3% agar. D. Quantification of colony formation assay from counting colonies from three random fields in three biological replicates. Colony defined as area >10,000µm2. Significance calculated using a paired t- test. E. Representative field (100x magnification) for cell invasion assay of A549-shScrambled and A549-shLINC00261 cells. F. Quantification of invasion assay from counting colonies from five random fields in three biological replicates. Statistical differences calculated using a paired t-test. G. Flow cytometric analysis of DNA content of LUAD cells using propidium iodide. Population was analyzed using FlowJo v10.1. N=3 (≥10,000 cells per sample). Representative FACS analysis shown. H. Quantification of flow analysis in (E). Three biological replicates representing isolated stable clones were quantified in technical triplicates.
Bioinformatic profiling and pathways analysis of LINC00261 targets
To determine the major pathways affected by LINC00261 function, we performed RNA-sequencing on the H522-CMV-NEO and H522-CMV-LINC00261 stable cell lines. RNA-seq analysis indicated that 108 genes were differentially expressed upon ectopic LINC00261 reintroduction (Figure 4A, B, Supplemental Table 3). Ingenutiy Pathways Analysis (IPA) revealed that the major pathways altered were G2/M DNA damage checkpoint signaling, GADD45 and RAN signaling (Figure 4C, blue bars). We then analyzed publicly available datasets to determine which genes and related pathways were significantly correlated to LINC00261 expression levels. To do so, we utilized the TANRIC database, which has calculated co-expression networks for differentially expressed lncRNAs in numerous cancers. 342 genes were significantly correlated with expression of LINC00261 in LUAD from TCGA RNA-seq profiling (P<1.0E-15, normals are excluded from this analysis). Performing enrichment analysis on those correlated genes indicated that G2/M cell cycle arrest and the DNA damage response (DDR) were the top correlated pathways (Figure 4C, purple bars). Strikingly, LINC00261 reintroducton caused an upregulation in mRNA levels of ATM kinase, TOP2A DNA helicase, and other critical members of the DDR pathway (Figure 4D). Many of the same genes had expression correlated to LINC00261 levels in TCGA LUAD gene expression profiling (Figure S5A-B).
Figure 4.
RNA-seq analysis of H522 CMV-NEO and CMV-LINC00261 stable cell lines reveals a role for LINC00261 in the DNA damage pathway response. A. Unsupervised hierarchical clustering using ward method for RPKMs of H522 CMV-NEO and CMV-LINC00261 stable cell lines. Top 5% most variant genes shown. B. Volcano plot of differential gene expression profiling. Red = upregulated in H522 CMV-LINC00261, green = downregulated in H522 CMV-LINC00261, as compared to H522 CMV-NEO control. C. IPA pathway enrichment of differentially expressed genes. Purple = correlated and anti-correlated genes with LINC00261 expression in TCGA LUAD as computed by TANRIC (45). Dark Blue = Differentially expressed genes between H522 CMV-NEO and CMV-LINC00261. Pathways are BH-corrected. D. IPA network analysis of significantly differentially expressed genes in the DNA damage response pathway with altered expression in the H522 CMV-LINC00261 RNAseq analysis. E. Western blotting of H522 CMV-NEO and H522 CMV-LINC00261 upon exposure to multiple doses of ATM inhibitor Ku-55933.
DNA damage pathway regulation is typically measured by the activation of protein phosphorylation. To determine if there was altered phosphorylation of key DDR members, we performed phospho-specific antibody staining on lysates from H522-CMV-LINC00261 alongside H522-CMV-NEO controls (Figure 4E). This demonstrated that reintroduction of LINC00261 was able to increase the amount of detectable ATM phosphorylation, as well as phosphorylation of CHK2, a downstream target of ATM. LINC00261 reintroduction also increased phosphorylation of BRCA2, as well as an increased total levels of ATR and BRCA1 protein (Figure S5C). Increased activation of ATM in the presence of LINC00261 was striking, as ATM is the sensor of DNA damage within the cell. To further assess if LINC00261 was acting upstream or downstream of ATM kinase we treated both CMV-NEO and CMV-LINC00261 cells with Ku-55933, a specific ATM inhibitor (36). Ku-55933 was able to block ATM phosphorylation as well as subsequent downstream phosporylation events, indicating that LINC00261 acts upstream of ATM activation to facilitate DNA damage response activation (Figure 4E). Therefore, LINC00261 may affect the ability of ATM to sense DNA damage and direct coordinated repair of damaged loci.
Regulation of LINC00261 by the pioneering transcription factor FOXA2
In searching for a mechanism by which LINC00261 expression is regulated in cancer, we discovered that LINC00261 is in close genomic proximity to the pioneering transcription factor FOXA2. This transcription factor has critical regulatory functions in prostate, lung, liver, and overall endoderm development. FOXA2 is essential for normal differentiation of the alveolar epithelium, as FOXA2 ablation results in disrupted alveolarization (37). To determine if FOXA2 plays a role in regulating LINC00261 expression, we performed a correlation analysis using co-expression from the TANRIC database. FOXA2 and LINC00261 expression were highly correlated in LUAD (R=0.91, p=3.5E-136) (Figure 5A). In addition, expression of LINC00261 was positively correlated to FOXA2 in several other epithelial cancers of endodermal origin, including hepatocellular carcinoma (R=0.66, P=03.3E-5), renal clear cell carcinoma (R=0.56, p=3.3E-38), prostate adenocarcinoma (R=.702, p= 4.1E-22), and lung squamous cell carcinoma (R=0.87, p=4.8E-71). Essentially all cancer types where LINC00261 was found to be significantly downregulated in tumor versus normal, comparisons were also highly correlated in expression to FOXA2. We then asked if this effect was due to regulation of LINC00261 expression by FOXA2. To do this, we introduced ectopic CMV-FOXA2 into H522 cells, which lack endogenous LINC00261 and FOXA2 expression. Ectopic reintroduction of FOXA2 stimulated expression of endogenous LINC00261 in trans (Figure 5B). However, when CMV-LINC00261 was introduced into H522 cells, we did not find a concomitant increase in FOXA2 expression (Figure 5C), suggesting unidirectional regulation. To determine if endogenous LINC00261 could affect FOXA2 levels as previously reported (38), we utilized A549 cells, which have endogenous expression of FOXA2 and LINC00261. ShRNA-mediated ablation of FOXA2 decreased endogenous expression of LINC00261 (Figure 5D). However, knockdown of LINC00261 did not affect FOXA2 levels (Figure 5E).
Figure 5.
FOXA2 regulates expression of LINC00261 in LUAD. A. Correlation between FOXA2 and LINC00261 expression from the TCGA LUAD dataset as computed by TANRIC (45). R2 = 0.91. B. qRT-PCR of FOXA2 and LINC00261 expression in H522 cells transiently transfected with CMV-FOXA2 or CMV-NEO control. Y axis is (−) Log2-fold change in expression between CMV-FOXA2 and CMV-NEO control. C. IGV image of BigWig tracks from H522-NEO and H522-CMV-LINC00261 RNA-seq data. All tracks are scaled 0-100 for read depth. Each row is an independent stable cell line. D. qRT-PCR of FOXA2 and LINC00261 expression in A549-shLINC00261 and A549-shScrambled controls. E. qRT-PCR of FOXA2 and LINC00261 expression in transiently transfected A549-shFOXA2 and A549-shScrambled controls. F. IGV image of cloned LINC00261 promoter relative to the LINC00261 transcription start site (promoter fragment is black). Grey = identified peaks from FOXA2 ChIPseq in A549 cells generated by ENCODE. G. Luciferase assay in A549 cells of LINC00261 promoter activity in the presence or absence of transiently transfected CMV-FOXA2. Luciferase values are normalized to total protein content and corrected for background pgl3 luciferase activity. RLU = Relative Light Units. A paired t-test was used to determine significance from four independent experiments, each with technical triplicates of plasmids derived from independent minipreps.
FOXA2 is known to act as a pioneering transcription factor. We therefore utilized ENCODE FOXA2 ChIPseq data in A549 cells to identify candidate FOXA2 transcription factor binding sites that may affect LINC00261 expression. We identified a FOXA2 binding site directly upstream of the LINC00261 transcription start site and cloned this region into the pgl3 promoter luciferase reporter construct (Figure 5F). Reporter cells containing the LINC00261 promoter construct and minimal promoter vector controls were transfected alongside CMV-FOXA2 and CMV-NEO plasmid controls. We observed a significant increase in overall promoter activity when comparing the pgl3 empty vector to the vector containing the LINC00261 promoter, indicating the promoter fragment was functional (p=0.0361) (Figure 5G). In addition, CMV-FOXA2 transfection was able to stimulate LINC00261 promoter activity significantly more than the empty vector control (p=0.0210) (Figure 5G). The level of induction seen for the LINC00261 promoter far exceeded the small increase observed for the reporter vector.
Epigenomic regulation of the FOXA2-LINC00261 locus
Because loss of FOXA2 and LINC00261 expression were correlated in LUAD, and they occupy the same genomic locus, we sought to identify if a common epigenetic mechanism of regulation was disrupted in cancer that could explain their mutual downregulation. We performed WGBS on purified AECs (27) and compared this to the WGBS profile of LUAD cell lines obtained from the Japanese database DBTSS. We observed that the FOXA2-LINC00261 locus contains a 25 kB unmethylated domain in normal AEC and this region shows extensive hypermethylation in the majority of tested LUAD cell lines (Figure 6A).
Figure 6.
DNA hypermethylation deactivates expression at the FOXA2/LINC00261 locus in LUAD. A. IGV track showing whole genome bisulfite sequencing (WGBS) of primary alveolar epithelial cells from three donor lungs and reduced representation bisulfite sequencing (RRBS) from DBTSS-generated LUAD cell lines. Red = methylated, blue = unmethylated, grey = non CpG sequence. B. Methylation of cg15058464 (LINC00261 promoter, hg19 chr20:22,559,803, −523 from TSS) and cg07003030 (FOXA2 promoter, hg19 chr20:22,565,995, −894 from TSS) in TCGA LUAD 450K array profiling (12) compared to adjacent tumor normal controls. C. Correlation between FOXA2 and Infinium 450K probe methylation in TCGA LUAD data for FOXA2 promoter (R2 = −0.56). D. Correlation between LINC00261 expression and Infinium 450K probe methylation in TCGA LUAD for the LINC00261 promoter (derived from cBioPortal, R2 = −0.41). E. Methylation levels derived from reduced representation bisulfite sequencing of A549 cells treated with 5-azaCdR. Image shows percent methylation of multiple treatment conditions across the LINC00261 promoter region. Green = CpG islands. F. Luciferase assay for activity of LINC00261 promoter in the presence or absence of in vitro SssI methylation. Data is normalized to CpGLess empty vector values from appropriate treatment group. A paired t-test was performed on N=3 biological replicates, each performed in technical triplicate.
While the WGBS results suggested that DNA methylation may play a role in regulation of the FOXA2-LINC00261 locus in LUAD, this experiment was performed on purified cells and cell lines. To determine if a similar correlation in DNA methylation was observed in primary tumors, we extracted the CpG methylation state and RNA expression levels from all LUAD TCGA tumors that had both data types available. Many of the CpG methylation sites surrounding the LINC00261 (Figure S6A) promoter region showed hypermethylation in LUAD; the most statistically significant of those is shown in Figure 6B. A significant negative correlation was observed between specific CpG methylation near the FOXA2 (Figure 6C) and LINC00261 (Figure 6D) promoters and their respective gene expression levels. Further examination of the DNMT family of enzymes implicates DNMT1 in the aberrant hypermethylation observed (Figure S6B).
Having established that hypermethylation was present at the FOXA2-LINC00261 locus, we sought to understand the functional consequences on FOXA2 and LINC00261 expression in LUAD. To test this, we extracted previously published reduced representation bisulfite sequencing information on A549 LUAD cancer cells treated for 13 days using multiple doses of 5-aza-cytidine (5-aza-CdR) (39). This drug incorporates into DNA during replication and binds irreversibly to one of the major DNA methylation transferases, DNMT1, effectively inducing global demethylation. We observed decreased methylation of the CpG island proximal to the LINC00261 transcription start site in the presence of 5-aza-CdR (Figure 6E). This effect was observed at both tested doses compared to vehicle controls. To determine if the CpG island methylation status within the FOXA2-LINC00261 locus could affect the transcriptional rate of LINC00261 and hypermethylation of FOXA2 promoter (Figure S7A), we subjected both LINC00261 and FOXA2 promoter constructs inserted into the CpGLess vector to in vitro SssI-mediated methylation. Vector backbone with and without SssI methylation were used as normalization controls. Methylated and unmethylated constructs were then transfected into A549 cells, and a significant decrease in LINC00261 promoter activity was observed (Figure 6F). Hypermethylation also reduced activity of the FOXA2 promoter, however these results did not meet the threshold for statistical significance (Figure S7B). These results suggest that hypermethylation of the FOXA2-LINC00261 locus decreases expression of both genes, resulting in a loss of downstream function.
Discussion
We have identified LINC00261 as a non-coding RNA with tumor suppressor characteristics in LUAD and describe mechanistically how LINC00261 could be responsible for the initial observations found in Liu et al (40). LINC00261 expression is lost in the vast majority of primary LUAD tumors and cell lines. Reintroduction of LINC00261 into LUAD cells was able to block proliferation, migration, and invasion capacity in vitro. Furthermore, we observed that LINC00261 is an integral part of the DNA damage response in lung cells, without which the cells are unable to effectively initiate G2/M cell cycle arrest and DNA damage repair signaling pathways critical for maintenance of healthy lung cells. In addition, we identified FOXA2 as a regulator of LINC00261 expression, the regulation of which is disrupted in LUAD by hypermethylation of the entire LINC00261-FOXA2 locus. Examination of RNAseq expression levels suggest that increased expression of DMNT1 may be responsible for the observed hypermethylation, however this would need to be further explored through detailed analysis of DNMT activity.
While our results point to the applicability of LINC00261 as a tumor suppressor in other forms of endodermally-derived epithelial lung cancers where FOXA2 is a critical transcription factor during development, we have not directly tested the effect of LINC00261 on carcinogenesis in other cancer types. Even when restricted to LUAD, there is controversy as to whether FOXA2 functions as a tumor suppressor, as conflicting data exists within the literature. Our results suggest that the tumor suppressive properties of FOXA2 may be mediated by its downstream target, LINC00261.
Many cancers exhibit global hypomethylation and localized hypermethylation in the CpG islands of gene promoters. However, many of these changes are thought to be passive events with little consequence on the overall proliferative and metastatic potential of the tumor (41). The observed epigenetic effect is interesting in the context of conflicting reports regarding the role of FOXA2 in lung cancer development. Many studies implicate FOXA2 as a tumor suppressor, equating this transcription factor as a differentiation signal that antagonizes cancer to help maintain the normal state. However, the actual mechanism by which FOXA2 exerts a tumor suppressing role is unknown, and there are also a few studies that demonstrate a pro-oncogenic role for FOXA2 in other epithelial cancers (42). Studies have evaluated the binding site affinity for FOXA2 (vs the homolog FOXA1) and seen enrichment in lipid metabolism genes, none of which were implicated in its effect on carcinogenesis (43). Demonstration of a functional role for CpG island methylation at this locus suggests a possible causal role for DNA methylation in tumorigenesis by deactivating LINC00261. Therefore, differential epigenetic silencing of LINC00261 in LUAD in specific contexts may resolve the confusion in the literature. Whether or not epigenetic silencing of the LINC00261-FOXA2 locus plays a role in other cancer types remains to be tested. Our results also suggest that epigenetic therapies such as 5-azacytidine may be a viable alternate strategy for the treatment of LUAD and other epithelial cancers, as this could reactivate LINC00261 and restore proper DNA damage response in these tumors.
We have therefore identified a lncRNA, LINC00261, that behaves as a tumor suppressor by blocking cellular proliferation through activation of the DNA damage signaling pathway to arrest cellular division. In addition, ATM, topoisomerase 2A (TOP2A), and other members of the DNA damage repair machinery have altered expression in the presence of LINC00261. Specifically, LINC00261 has been reported to alter the efficacy of cisplatin therapy in colon cancer (44). Our study provides a mechanistic basis for this observation. In identifying a critical regulatory component of the DNA damage response, we have uncovered a new aspect of this critical pathway in carcinogenesis, one that opens up the possibility for development of novel chemotherapeutic agents targeting this lncRNA to treat this deadly disease.
Supplementary Material
Acknowledgements:
We thank Dr. Ite A. Offringa for providing oversight and motivation to the project, as well as the laboratory of Dr. Omid Akbari for assistance with cell cycle analysis. In addition, we thank Charlie Nicolet, PhD, and the USC Epigenome Center for generation of all sequencing data as well as Suhn Rhie, PhD for assistance in manipulating the publicly available DBTSS whole genome bisulfite sequencing data for easy visualization. Funding for the project was provided by the USC Department of Surgery Research Fund, the Howard and Andree Shore STOP cancer research fund, the Baxter Foundation, and the Wright Foundation. This work was supported by in part by the Norris Comprehensive Cancer Center core grant, award number P30CA014089, from the National Cancer Institute. BZ received support from the Hastings and Whittier Foundations, the Will Rogers Institute, and research grant HL114959 from the National Institutes of Health. ZB received support from the Hastings and Whittier Foundations, the Will Rogers Institute, and research grant R35 HL135747 from the National Institutes of Health. ZB is Ralph Edgington Chair in Medicine and Hastings Professor of Medicine.
Footnotes
The authors declare no potential conflicts of interest
References
- 1.Ridge CA, McErlean AM, Ginsberg MS. Epidemiology of lung cancer. Semin Intervent Radiol. 2013;30(2):93–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Humphrey LL, Deffebach M, Pappas M, Zakher B, Slatore CG. Screening for lung cancer with low-dose computed tomography. Ann Intern Med. 2014;160(3):212. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z, Fillmore CM, Hammerman PS, Kim CF, Wong KK. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer. 2014;14(8):535–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal S Targeted cancer therapies. Nat Rev Drug Discov. 2010;9(6):427–8. [DOI] [PubMed] [Google Scholar]
- 6.Vanneman M, Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer. 2012;12(4):237–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng L, Alexander RE, Maclennan GT, Cummings OW, Montironi R, Lopez-Beltran A, et al. Molecular pathology of lung cancer: key to personalized medicine. Mod Pathol. 2012;25(3):347–69. [DOI] [PubMed] [Google Scholar]
- 8.Siegelin MD, Borczuk AC. Epidermal growth factor receptor mutations in lung adenocarcinoma. Lab Invest. 2014;94(2):129–37. [DOI] [PubMed] [Google Scholar]
- 9.Hata A, Katakami N, Yoshioka H, Fujita S, Kunimasa K, Nanjo S, et al. Erlotinib after gefitinib failure in relapsed non-small cell lung cancer: clinical benefit with optimal patient selection. Lung Cancer. 2011;74(2):268–73. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352(8):786–92. [DOI] [PubMed] [Google Scholar]
- 11.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2(3):e73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Network CGAR. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo JS, Ju YS, Lee WC, Shin JY, Lee JK, Bleazard T, et al. The transcriptional landscape and mutational profile of lung adenocarcinoma. Genome Res. 2012;22(11):2109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derrien T, Johnson R, Bussotti G, Tanzer A, Djebali S, Tilgner H, et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vance KW, Ponting CP. Transcriptional regulatory functions of nuclear long noncoding RNAs. Trends Genet. 2014;30(8):348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercer TR, Mattick JS. Structure and function of long noncoding RNAs in epigenetic regulation. Nat Struct Mol Biol. 2013;20(3):300–7. [DOI] [PubMed] [Google Scholar]
- 17.Geisler S, Coller J. RNA in unexpected places: long non-coding RNA functions in diverse cellular contexts. Nat Rev Mol Cell Biol. 2013;14(11):699–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Network CGAR. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunner AL, Beck AH, Edris B, Sweeney RT, Zhu SX, Li R, et al. Transcriptional profiling of long non-coding RNAs and novel transcribed regions across a diverse panel of archived human cancers. Genome Biol. 2012;13(8):R75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang C, Wang X, Li X, Zhao N, Wang Y, Han X, et al. The landscape of DNA methylation-mediated regulation of long non-coding RNAs in breast cancer. Oncotarget. 2017;8(31):51134–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz-Lagares A, Crujeiras AB, Lopez-Serra P, Soler M, Setien F, Goyal A, et al. Epigenetic inactivation of the p53-induced long noncoding RNA TP53 target 1 in human cancer. Proc Natl Acad Sci U S A. 2016;113(47):E7535–E44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108(12):2419–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iyer MK, Niknafs YS, Malik R, Singhal U, Sahu A, Hosono Y, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet. 2015;47(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castillo J, Stueve TR, Marconett CN. Intersecting transcriptomic profiling technologies and long non-coding RNA function in lung adenocarcinoma: discovery, mechanisms, and therapeutic applications. Oncotarget. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishikawa Y, Kozakai T, Morita H, Saida K, Oka S, Masuo Y. Rapid detection of mycoplasma contamination in cell cultures using SYBR Green-based real-time polymerase chain reaction. In Vitro Cell Dev Biol Anim. 2006;42(3–4):63–9. [DOI] [PubMed] [Google Scholar]
- 27.Marconett CN, Zhou B, Rieger ME, Selamat SA, Dubourd M, Fang X, et al. Integrated transcriptomic and epigenomic analysis of primary human lung epithelial cell differentiation. PLoS Genet. 2013;9(6):e1003513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stueve TR, Li WQ, Shi J, Marconett CN, Zhang T, Yang C, et al. Epigenome-wide analysis of DNA methylation in lung tissue shows concordance with blood studies and identifies tobacco smoke-inducible enhancers. Hum Mol Genet. 2017;26(15):3014–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Justus CR, Leffler N, Ruiz-Echevarria M, Yang LV. In vitro cell migration and invasion assays. J Vis Exp. 2014(88). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gebäck T, Schulz MM, Koumoutsakos P, Detmar M. TScratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotechniques. 2009;46(4):265–74. [DOI] [PubMed] [Google Scholar]
- 31.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2(2):329–33. [DOI] [PubMed] [Google Scholar]
- 32.Tomayko MM, Reynolds CP. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother Pharmacol. 1989;24(3):148–54. [DOI] [PubMed] [Google Scholar]
- 33.White NM, Cabanski CR, Silva-Fisher JM, Dang HX, Govindan R, Maher CA. Transcriptome sequencing reveals altered long intergenic non-coding RNAs in lung cancer. Genome Biol. 2014;15(8):429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park C, Yu N, Choi I, Kim W, Lee S. lncRNAtor: a comprehensive resource for functional investigation of long non-coding RNAs. Bioinformatics. 2014;30(17):2480–5. [DOI] [PubMed] [Google Scholar]
- 35.Cao WJ, Wu HL, He BS, Zhang YS, Zhang ZY. Analysis of long non-coding RNA expression profiles in gastric cancer. World J Gastroenterol. 2013;19(23):3658–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stiff T, Walker SA, Cerosaletti K, Goodarzi AA, Petermann E, Concannon P, et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 2006;25(24):5775–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wan H, Xu Y, Ikegami M, Stahlman MT, Kaestner KH, Ang SL, et al. Foxa2 is required for transition to air breathing at birth. Proc Natl Acad Sci U S A. 2004;101(40):14449–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jiang W, Liu Y, Liu R, Zhang K, Zhang Y. The lncRNA DEANR1 facilitates human endoderm differentiation by activating FOXA2 expression. Cell Rep. 2015;11(1):137–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hascher A, Haase AK, Hebestreit K, Rohde C, Klein HU, Rius M, et al. DNA methyltransferase inhibition reverses epigenetically embedded phenotypes in lung cancer preferentially affecting polycomb target genes. Clin Cancer Res. 2014;20(4):814–26. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Xiao N, Xu SF. Decreased expression of long non-coding RNA LINC00261 is a prognostic marker for patients with non-small cell lung cancer: a preliminary study. Eur Rev Med Pharmacol Sci. 2017;21(24):5691–5. [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez-Arcelus M, Lappalainen T, Montgomery SB, Buil A, Ongen H, Yurovsky A, et al. Passive and active DNA methylation and the interplay with genetic variation in gene regulation. Elife. 2013;2:e00523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang B, Liu G, Ding L, Zhao J, Lu Y. FOXA2 promotes the proliferation, migration and invasion, and epithelial mesenchymal transition in colon cancer. Exp Ther Med. 2018;16(1):133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bochkis IM, Schug J, Ye DZ, Kurinna S, Stratton SA, Barton MC, et al. Genome-wide location analysis reveals distinct transcriptional circuitry by paralogous regulators Foxa1 and Foxa2. PLoS Genet. 2012;8(6):e1002770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang ZK, Yang L, Wu LL, Mao H, Zhou YH, Zhang PF, et al. Long non-coding RNA LINC00261 sensitizes human colon cancer cells to cisplatin therapy. Braz J Med Biol Res. 2017;51(2):e6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Han L, Roebuck P, Diao L, Liu L, Yuan Y, et al. TANRIC: An Interactive Open Platform to Explore the Function of lncRNAs in Cancer. Cancer Res. 2015;75(18):3728–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Győrffy B, Surowiak P, Budczies J, Lánczky A. Online survival analysis software to assess the prognostic value of biomarkers using transcriptomic data in non-small-cell lung cancer. PLoS One. 2013;8(12):e82241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.