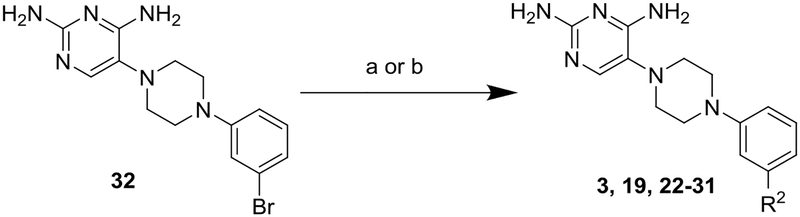
Scheme 2.
aSynthesis of Compounds 3, 19, 22–31
R2 = (o) 5-(2-methoxypyrimidine), (p) cyclopropyl, (q) 3-pyridine, (r) 4-pyridine, (s) 5-pyrimidine, (t) 4-pyridazine, (u) 5-(2-methylpyrimidine), (v) 5-(2-trifluoromethylpyrimidine), (w) 5-(2-cyclopropylpyrimidine), (x) 2-pyridine, (y) 2-pyrimidine and (z) 2-pyrazine.
a Reagents and conditions: (a) Pd(PPh3)4, R2-B(OH)2 (39o-w), Cs2CO3, Dioxane/H2O, 110 °C; or (b) Pd2(dba)3, Sn(nBu)3-R2 (40x-z), Xphos, dioxane, 100 °C