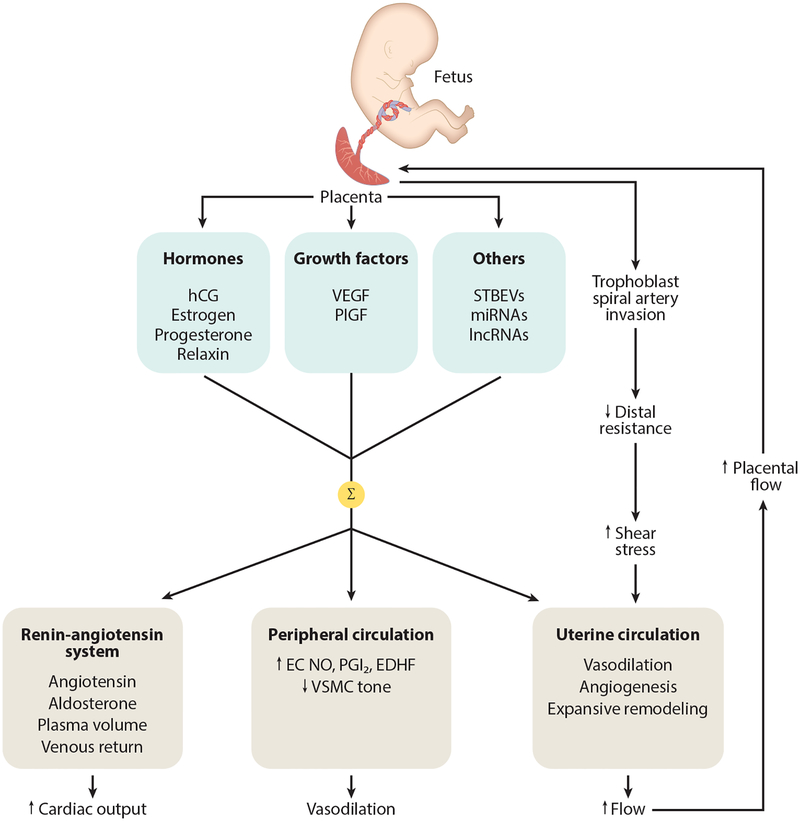
Figure 1.
Diagram showing some of the cardiovascular adaptations a woman experiences during pregnancy. The placenta secretes a variety of molecular signals (hormones, growth factors, others) into the maternal circulation. Their summative effects (Σ) result in activation of the renin-angiotensin system that acts to increase aldosterone, augmenting sodium and water reabsorption from the kidney in order to expand plasma volume; other components of the blood (cells, proteins) also increase but not as much as plasma volume, resulting in some hemodilution (not shown). Peripheral vasodilation is accomplished through increased EC release of NO, PGI2, and EDHF along with reduced VSMC myogenic tone. This leads to a reduction in peripheral resistance, which helps to accommodate the increased blood volume and maintain blood pressure at normotensive (or even slightly reduced) levels. In addition to vasodilation, the uterine circulation undergoes angiogenesis and expansive remodeling. These processes are stimulated by increased arterial wall shear stress that results from hemochorial placentation, which through trophoblast invasion and remodeling of spiral arteries, decreases distal resistance and accelerates blood in upstream vessels. The uterine circulation, uterus, and placenta grow in parallel, and uteroplacental blood flow increases progressively during pregnancy to levels that, in women, approach 1 L/min at term. Abbreviations: EC, endothelial cell; EDHF, endothelium-derived hyperpolarizing factor; hCG, human chorionic gonadotropin; lncRNA, long ncRNA; miRNA, microRNA; ncRNA, noncoding RNA; NO, nitric oxide; PGI2, prostacyclin; PlGF, placental growth factor; STBEV, syncytiotrophoblast-derived extracellular vesicle; VEGF, vascular endothelial growth factor; VSMC, vascular smooth muscle cell.