Figure 1: Protein secretion and export in Acinetobacter baumannii.
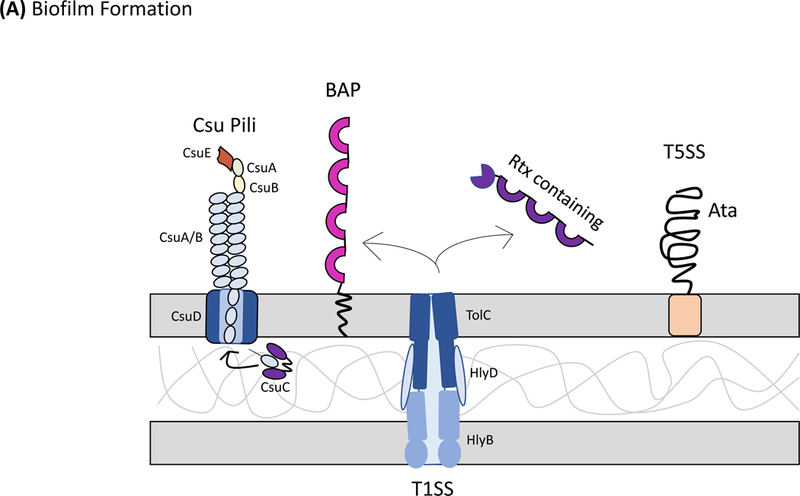
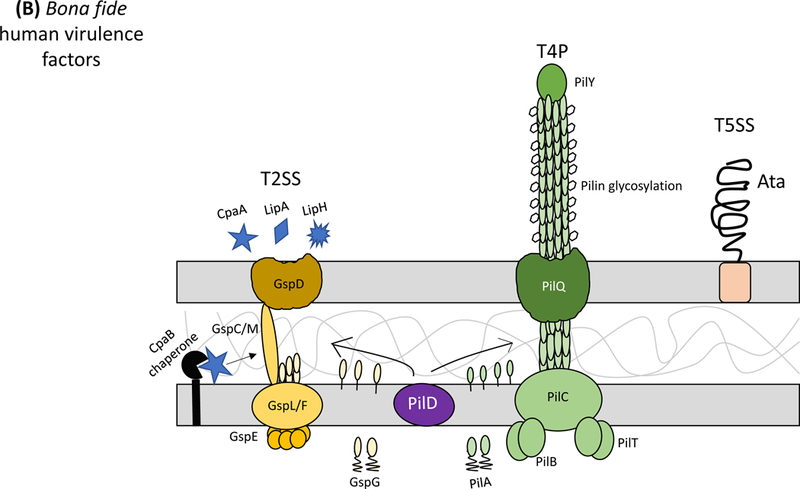
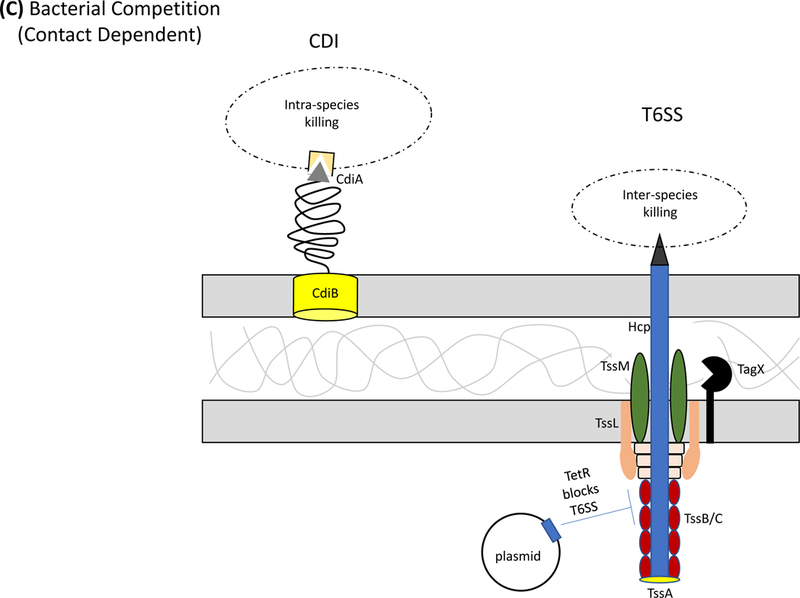
Secretion systems and extracellular appendages of Acinetobacter baumannii are involved in the formation of biofilms, in virulence and bacterial competition. (A) The Csu system extrudes a type I chaperone-usher pilus that is essential for the formation and maintenance of biofilms on abiotic surfaces, which may contribute to persistence in the hospital environments43. The CsuA/B pilin subunits are trafficked to the outer membrane CsuD usher by CsuC chaperones. The CsuD usher facilitates polymerization of CsuA/B monomers into a pilin fiber. Pilin polymerization is initiated by the CsuE tip adhesion and the minor pilins CsuA and CsuB, all of which are also trafficked to the CsuD usher by CsuC chaperones. Biofilm-associated proteins (Bap)50 and an effector protein that contains an RTX-like domain are secreted by the type I secretion system (T1SS)52 and are involved in the formation and stability of mature biofilms. The T1SS is composed of the outer membrane protein TolC, the periplasmic adaptor protein HlyD, and the inner membrane ATPase HlyB. The Acinetobacter trimeric autotransporter (Ata) type Vc secretion system consists of a membrane-associated transporter domain and a large, repetitive passenger domain that extrudes through the transporter domain60. The adhesin promotes adherence to extracellular and basal membrane components of the host. (B) The type II secretion system (T2SS) secretes multiple effectors that were shown to be required for virulence in vivo, including the lipases LipA and LipH as well as the protease CpaA103,104. Novel, dedicated chaperones are required for two of the effectors, including the CpaB chaperone, which is required for the secretion of CpaA, the most abundant type II effector. A. baumannii and A. nosocomialis also produce type IV pili, surface appendages evolutionarily related to the T2SS66. In A. baumannii and A. nosocomialis these two systems share a processing protein, PilD, required to process pre-pseudopilins and pre-pilins prior to assembly into the T2SS and type IV pilus, respectively104. Type IV pili are also glycosylated in A. baumannii and A. nosocomialis as a possible form of antigenic variation to evade host detection. Specifically, the pilin glycan is predicted to mask the major pilin subunit, PilA, from antibody recognition; furthermore, PilA displays remarkable sequence divergence across species and even between strains leading to reduced immune recognition in the case pilin subunits are not glycosylated68. (C) Contact-dependent secretion systems are used by A. baumannii for inter- and intra-species killing to eliminate bacterial competitors. Many strains of Acinetobacter have two distinct contact-dependent inhibition (CDI) systems to kill sister cells that do not have the associated immunity protein52. The type VI secretion system (T6SS)106,107 is used for inter-species competition and contains a novel L,D-endopeptidase, TagX, which is required for transit of the machinery through the peptidoglycan layer128. A large, conjugative plasmid which also contains drug-resistance genes (not indicated) regulates the expression of T6SS in some clinical isolates110. The individual components of the secretion systems are indicated in all panels.
