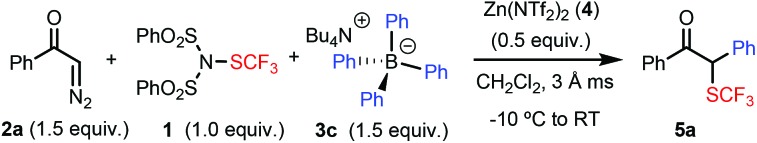
Table 1. Deviation from the optimal reaction conditions for the α,α′-trifluoromethylthiolation–phenylation of diazoketone 2a a .
| ||
| Entry | Deviation from the standard conditions | Yield b (%) |
| 1 | None | 81 |
| 2 | 0.25 equiv. Zn(NTf2)2 (4) | 37 |
| 3 | Without Zn(NTf2)2 (4) | <5 |
| 4 | Rh2(OAc)4 (5 mol%) instead of 4 | 0 |
| 5 | Pd(OAc)2 (15 mol%) instead of 4 | 30 |
| 6 | Zn(OTf)2 instead of 4 | <5 |
| 7 | 0.5 equiv. of 3c | 28 |
| 8 | Na(BPh4) instead of 3c | 52 |
| 9 | (PhBO)3 (3a) instead of 3c, without 4 | 0 |
| 10 | BPh3 (3b) instead of 3c, without 4 | 15 |
| 11 | ZnPh2 instead of 3c | <5 |
| 12 | ZnPh2 instead of 4 and BPh33b instead of 3c | 0 |
| 13 | PhMe as the solvent | 71 |
| 14 | THF as the solvent | 35 |
| 15 | MeCN as the solvent | 0 |
| 16 | Without 3Å ms | 59 |
| 17 | 22 °C instead of –10 °C | 66 |
aTo reagent 1 (0.1 mmol), Bu4N(BPh4) (3c) (0.15 mmol), Zn(NTf2)2 (4) (0.05 mmol) and 80 mg 3Å molecular sieves (ms) was added a solution of diazoketone 2a (0.15 mmol) in CH2Cl2 (1.0 ml) at –10 °C. This mixture was stirred at –10 °C for 2 h before allowing it to warm up to RT overnight.
bIsolated yield.
