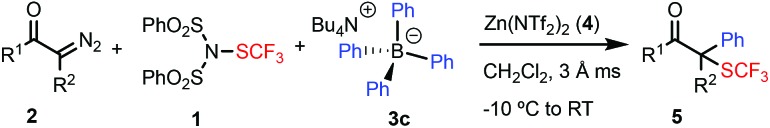
Table 3. 1,1-Trifluoromethylthiolation–phenylation of diazo compounds 2 with SCF3-source 1 and Bu4N(BPh)4 (3a) a .
| |||
| Entry | Diazocarbonyl compound 2 | Carbonyl compound 5 | Yield b of 5 (%) |
| 1 |
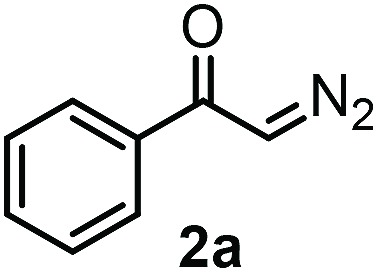
|
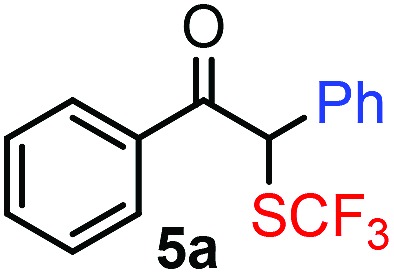
|
81 |
| 2 |
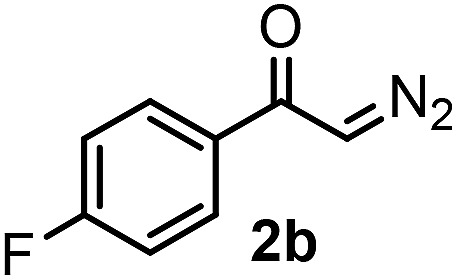
|
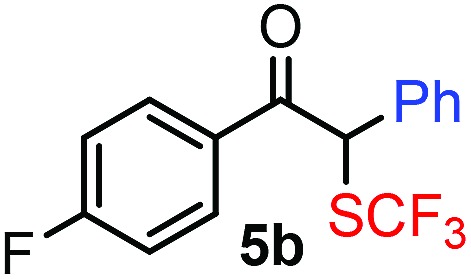
|
80 |
| 3 |
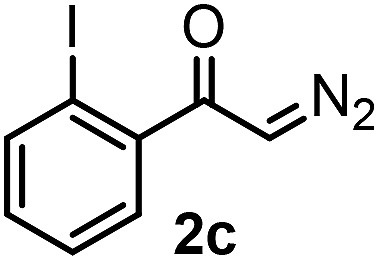
|
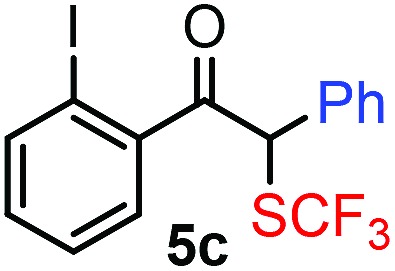
|
73 |
| 4 |
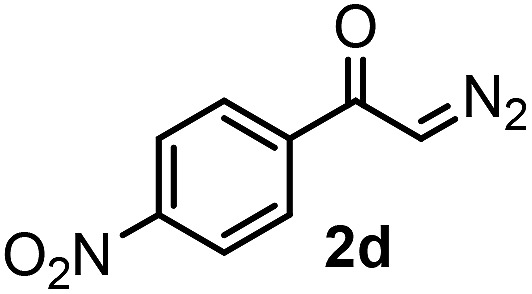
|
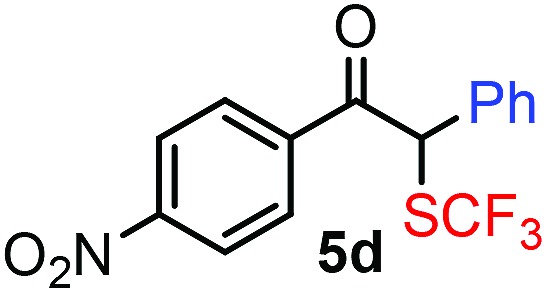
|
84 |
| 5 |
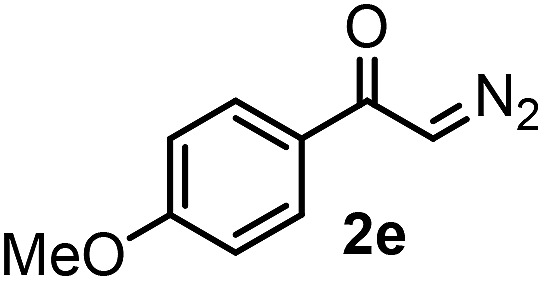
|
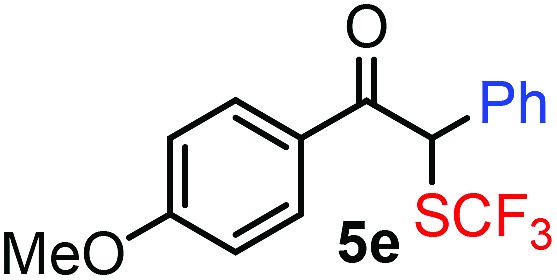
|
60 |
| 6 |
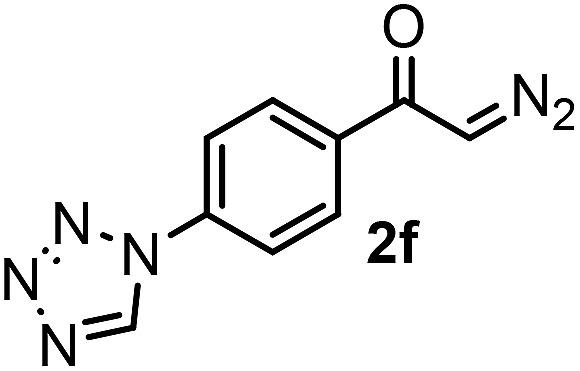
|
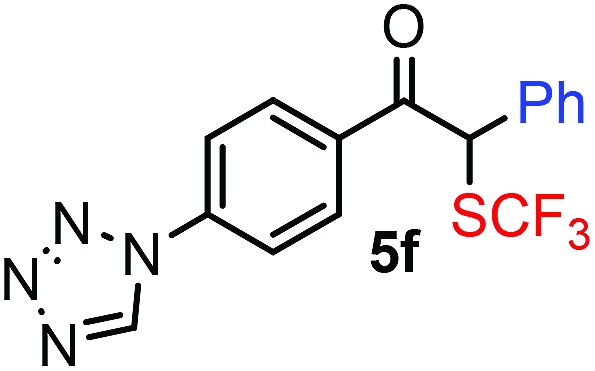
|
68 c |
| 7 |
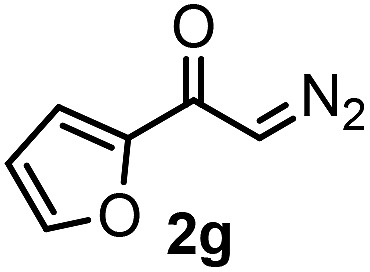
|
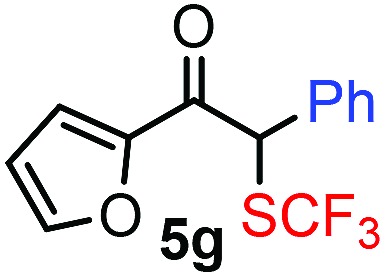
|
84 |
| 8 |
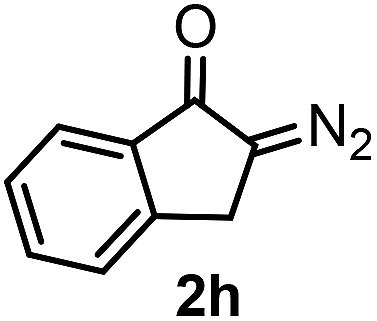
|
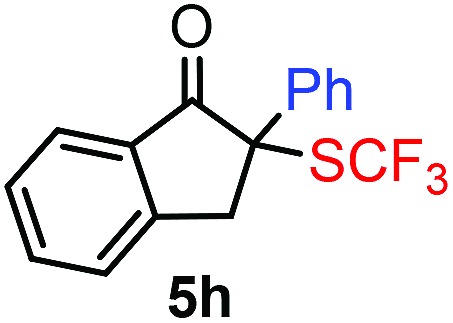
|
30 c |
| 9 |
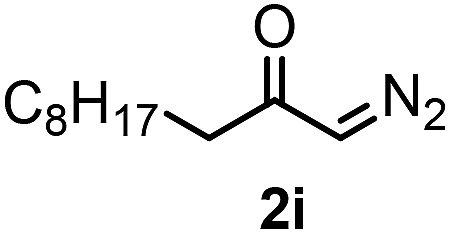
|
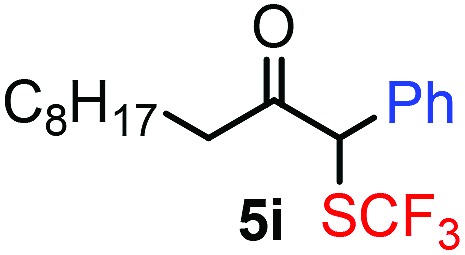
|
83 |
| 10 |
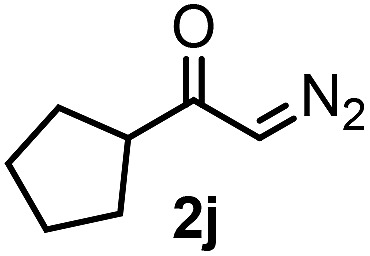
|
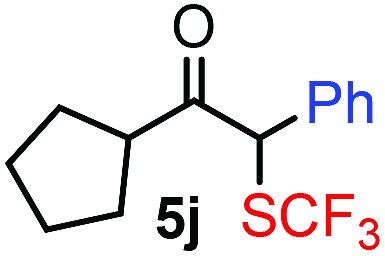
|
48 |
| 11 |
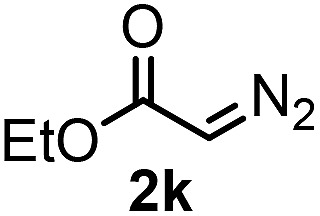
|
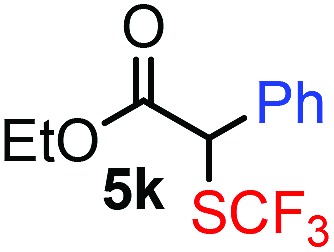
|
78(74) d |
| 12 |
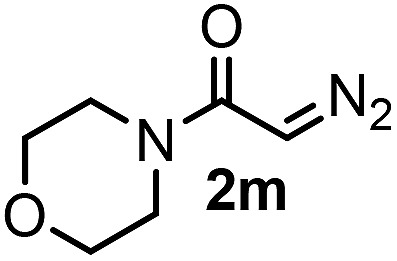
|
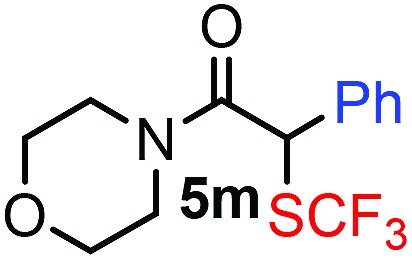
|
80 |
| 13 |
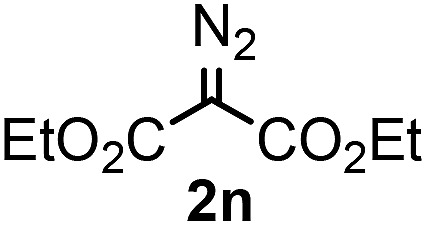
|
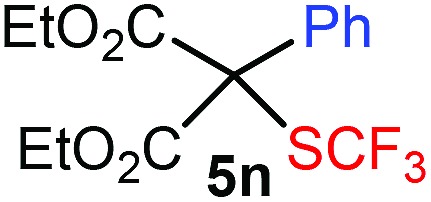
|
0 |
| 14 |
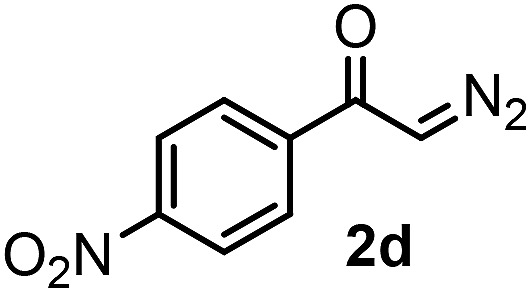
|
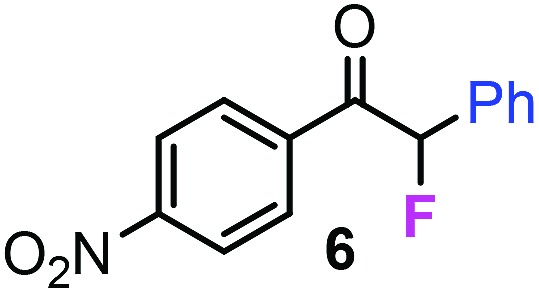
|
55 c , e , f |
aUnless otherwise stated: to 1 (0.1 mmol), Bu4N(BPh4) (3c) (0.15 mmol), Zn(NTf2)2 (4) (0.05 mmol) and 3Å ms (80 mg) was added a solution of 2 (0.15 mmol) in CH2Cl2 (1.0 ml) at –10 °C, stirred for 2 h before warmed up to RT overnight.
bUnless otherwise stated isolated yield.
cRT overnight.
d1.0 mmol scale.
eInstead of 1, NFSI was used.
fYield of 6 (determined by 1H-NMR analysis) contains side product 1-(4-nitrophenyl)-2-phenylethan-1-one.
