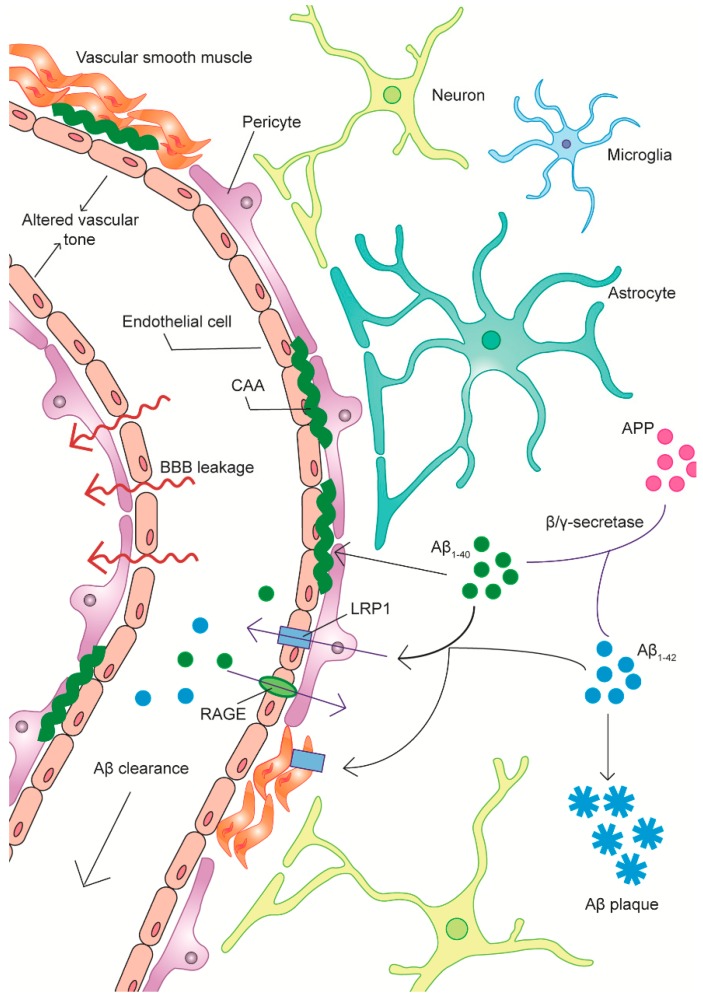
Figure 1.
Dysregulated Amyloid-β (Aβ) Clearance in Alzheimer’s Disease (AD). The vasculature is the site of a complex amyloid-β clearance system. Pathological Aβ species, including Aβ1-40 and Aβ1-42, are generated by the cleavage of the amyloid precursor protein (APP) by the enzyme β-secretase and the subsequent cleavage of the soluble amyloid precursor protein-α (sAPPα) product by γ-secretase. Aβ binds to low density lipoprotein receptor-related protein-1 (LRP1) on the abluminal membranes of vascular cells and LRP1 mediates the internalization of the peptide by an endocytotic pathway, thus aiding in Aβ clearance and removal from the brain. The receptor for advanced glycation endproducts (RAGE), on the other hand, is involved in the transport of free Aβ from the systemic circulation into the brain. In AD, Aβ clearance mechanisms are impaired, potentially at an early stage. This includes the downregulation of LRP1 and the upregulation of RAGE in AD microvessels. Reduced Aβ clearance may contribute to Aβ deposition as parenchymal senile plaques or vascular deposits. Vascular Aβ deposition may progress to the development of cerebral amyloid angiopathy (CAA) in capillaries as well as in the smooth muscle layers of arterioles. Aβ peptides exert toxic effects on vascular cells, contribute to the dysregulation of vascular tone, induce vascular inflammation and contribute to the weakening of the blood-brain barrier. Thus, excess Aβ is involved in several mechanisms of vascular dysfunction in AD, which can also have serious consequences for disease risk and progression.