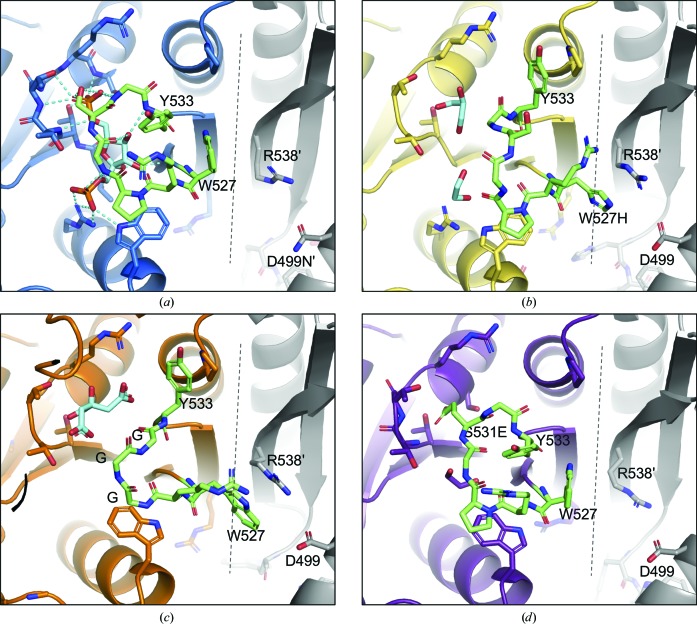
Figure 3.
Comparison of the allosteric loop. The allosteric loop is colored green. Ligands are colored cyan. A dashed line marks the C–C interface. Adjacent monomers are colored gray. (a) The D499N variant. The D499N variant has a closed allosteric loop with fructose-1,6-bisphosphate (Fru-1,6-BP) bound. (b) The W527H variant. The allosteric loop is in an open position. W527H exhibits a cation–π interaction with Arg538 across the C–C interface, stabilizing the conformation. (c) The GGG variant with an open allosteric loop. Trp527 forms a π-stacking interaction with Arg538. (d) The S531E variant with a closed allosteric loop. The Glu531 carboxylate is bound in the position of the 6′-phosphate of Fru-1,6-BP, stabilizing the closed conformation.