Table 5.
Conversion of 4-(5-amino-4-cyano-1H-pyrazol-1-yl)-benzoic acid (1u) to 4-(4-cyano-1H-pyrazol-1-yl)-benzoic acid (2u) with various quantities of gallic and salicylic acid.
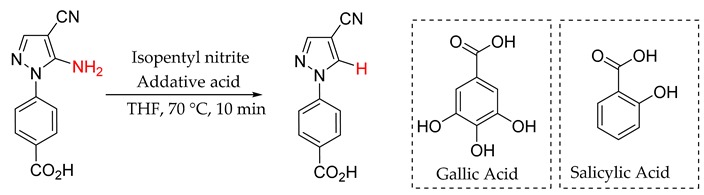
| ||
|---|---|---|
| Catalyst Loading (mol%) | Conversion (%) Gallic Acid | Conversion (%) Salicylic Acid |
| 0 | 0 | 0 |
| 1 | 0 | 0 |
| 5 | 33 | 1 |
| 10 | 26 | 26 |
| 10a | 18 | 22 |
| 20 | 22 | 30 |
| 100 | 0 | 21 |
All experiments were: 4-(5-amino-4-cyano-1H-pyrazol-1-yl)-benzoic acid (1.0 mmol) and the stated quantity of gallic acid or salicylic acid in THF and iso-pentyl nitrite (1.2 mmol) in THF (50 mL), flow rate 1.0 mL min−1, through a 10 mL coil reactor maintained at 70 °C, giving a residence time of 10 min. The conversions were calculated by integration of product 1H-NMR peaks relative to a quantified internal standard of nitrobenzene. aReaction performed at 120 °C.
