Figure 1.
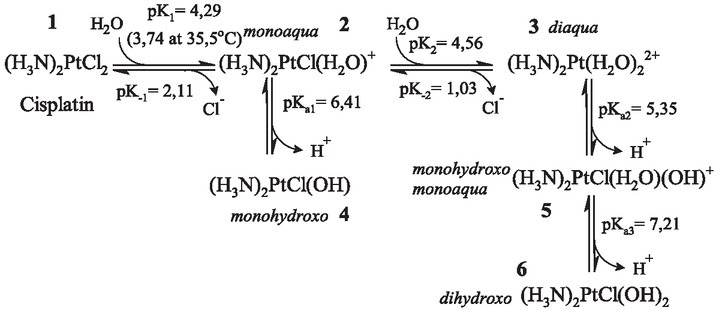
The behaviour of cisplatin in aqueous solution. Cisplatin (1) is a bright-yellow solid; when dissolved in water it is attacked by water molecules and as a result one of the chloride ions is eliminated and monoaqua (2) species is formed. Diaqua species (3) is formed when the second water molecule replaces the chloride ion. Water replaces chloride ions because the metal and nitrogen form stronger bond than metal and the chloride ion. Bound water became very acidic and at physiologic pH became completely deprotonated – as a monohydroxo form (4), and the product of the dissociation of the second proton from the diaqua form is dihydroxo species (6). Logarithms of rate constants (pK1, pK-1, pK2 and pK-2) are given for 25 ºC and of dissociation constants (pKa1, pKa2 and pKa3) for 27 ºC.
