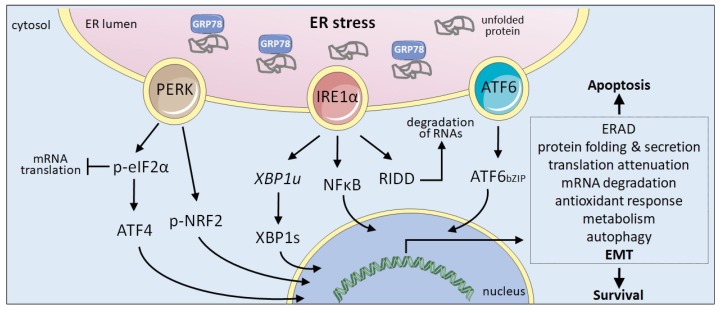
Figure 1.
The UPR. The ER protein maturation capacity may be overwhelmed due to the action of several cell intrinsic and extrinsic factors, causing ER stress. The accumulation of unfolded proteins triggers the activation of the three ER-resident sensors responsible for UPR by sequestering GRP78. IRE1 mediates the unconventional splicing of the mRNA encoding XBP1 (XBP1u) rendering the functional transcription factor XBP1s and can activate NFκB signalling. IRE1 RNase degrades ER associated RNAs through RIDD (regulated IRE1-dependent decay). PERK phosphorylates eIF2α to inhibit global translation while promoting the translation of the transcription factor ATF4. PERK can also phosphorylate NRF2. ATF6 is exported from the ER to the Golgi apparatus, were the SP1 and SP2 proteases mediate the release of the bZIP domain (ATF6bZIP). In the nucleus, XBP1s, ATF4 and ATF6bZIP transcription factors trigger the expression of a large number of genes to help cells alleviate ER stress. Upon persistent ER stress, UPR favours apoptosis. Cancer cells exploit UPR signalling to promote survival under tumour-associated stress situations.