Table 1.
Evaluation of Reaction Conditions for the CuH-Catalyzed Allylation of 4-Methoxyacetophenone.a
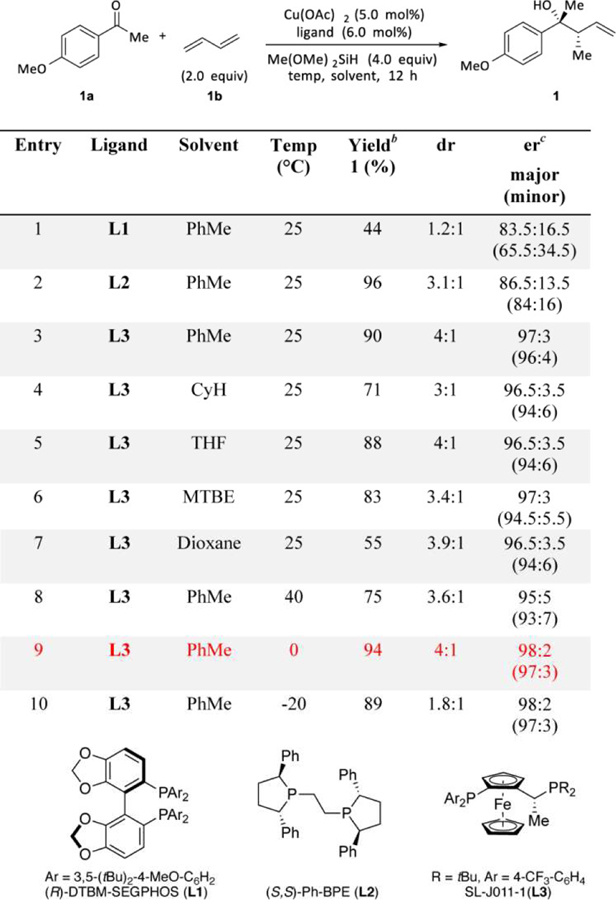 |
Conditions: 0.2 mmol ketone (1 equiv), 1,3-butadiene (2 equiv), copper(II) acetate (0.05 equiv), ligand (0.06 equiv), dimethoxy(methyl)silane (4 equiv) in solvent (0.2 mL), ketone was added slowly by syringe pump; see the Supporting Information for details.
Yield and diastereomeric ratio were determined by 1H NMR spectroscopy of the crude mixture, using dibromomethane as an internal standard.
Enantiomeric ratio was determined by HPLC or SFC analysis on commercial chiral columns, and the relative configuration of 1 was determined by comparing its NMR data with reported data.17
