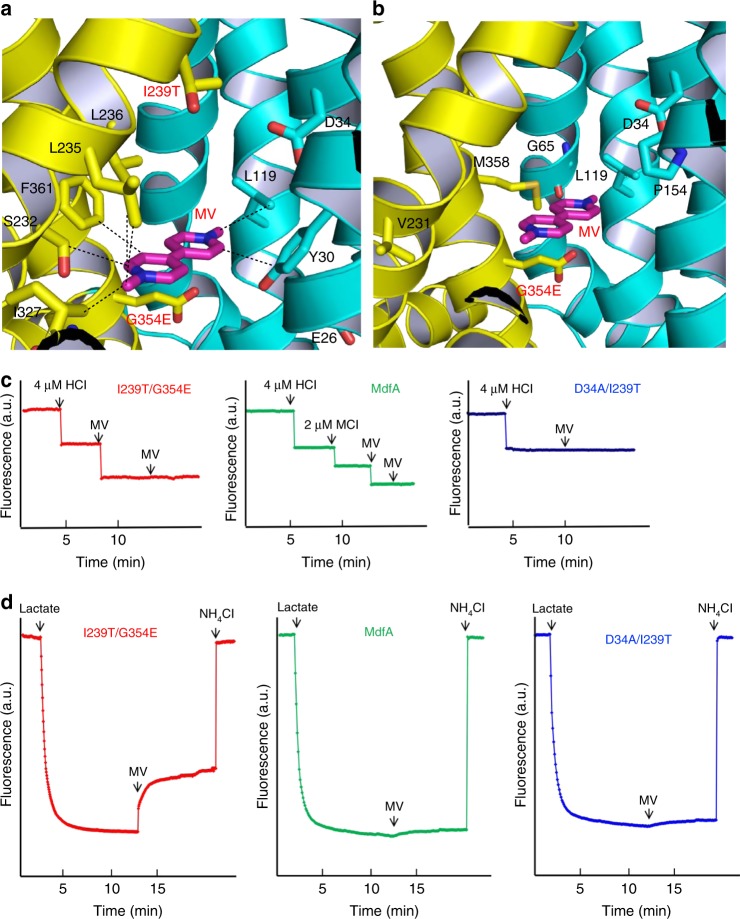
Fig. 4.
Recognition and extrusion of MV by I239T/G354E. a Close-up view of the MV-binding site in I239T/G354E, with the N and C domains colored cyan and yellow, respectively. The bound MV is drawn in stick models and colored magenta. The relevant amino acids are shown in stick models and close-range interactions are highlighted by dashed lines. b Location of the mutated amino acids that resulted in the new functionality of transporting short dicationic compounds. The bound MV (magenta) and relevant amino acids are shown in stick models. c Fluorescence measurement of a solution containing 2 µM I239T/G354E (red) or MdfA (green), revealing its ability to release two or one proton per protein molecule upon MV binding. As a comparison, the addition of MV to a solution containing 2 µM D34A/I239T (blue), failed to trigger the release of H+. d MV/H+ antiport observed in the everted membrane vesicles expressing I239T/G354E (red). H+ movement was monitored by measurement of acridine orange fluorescence, which is shown in arbitrary units (a.u.). By contrast, MV failed to trigger H+ movement in the everted membrane vesicles harboring MdfA (green) or D34A/I239T (blue). The traces are representative of experiments performed in triplicate using two different preparations of everted membrane vesicles