Fig. 1.
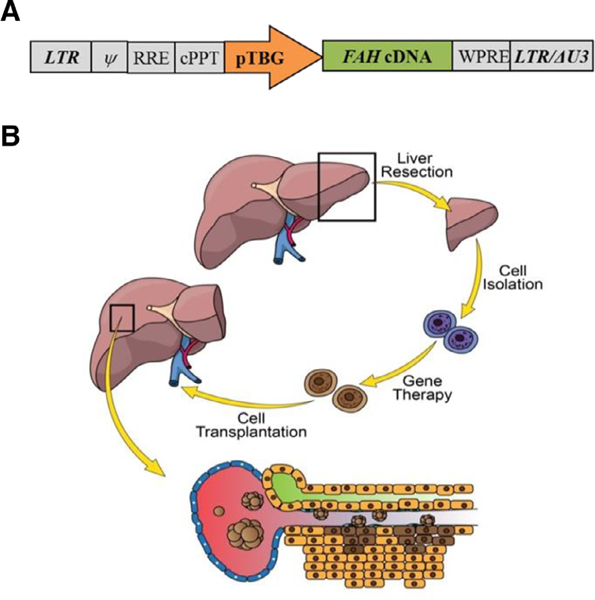
Second-generation lentiviral vector and experimental process involved in ex vivo gene therapy. (A) The porcine Fah cDNA is under control of the human TBG promoter. LTR, long terminal repeat; Ψ, psi packaging sequence; RRE, rev-responsive element; cPPT, central polypurine tract; WPRE, woodchuck hepatitis virus posttranscriptional regulatory element; LTR/∆U3, 3´ long terminal repeat with deletion in U3 region. (B) Four steps are involved in this process: (1) partial hepatectomy, (2) primary hepatocyte isolation through collagenase perfusion, (3) ex vivo gene therapy with a lentiviral vector carrying the porcine Fah gene, and (4) retransplantation of autologous hepatocytes in single cell or spheroid form.
