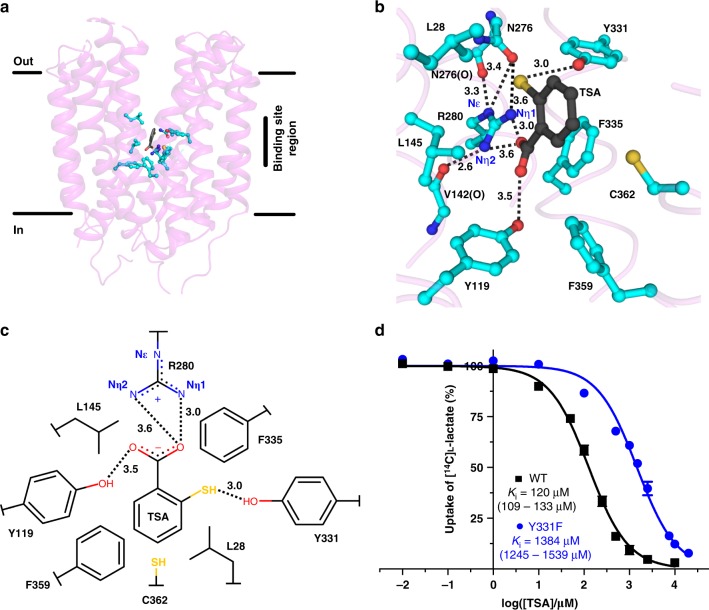
Fig. 3.
Binding pocket of SfMCT with bound TSA. a Overall structure of SfMCT in the outward-open conformation viewed in the plane of the membrane with indicated TSA (black) and binding site residues (cyan). b Binding mechanism of TSA to SfMCT. Residues within a distance of 4 Å from TSA, N276 and the peptide backbone of V142 are displayed as ball-and-stick model and are highlighted in cyan. c Two-dimensional schematic representation of key residues that are involved in TSA-binding. Distances in (b, c) are given in Ångström (Å). d Ki determination of wild-type (WT) and Y331F SfMCT for TSA. The determined Ki values are 120 μM (95% confidence interval: 109–133 μM) for WT and 1384 μM (95% confidence interval: 1245–1539 μM) for Y331F. Data are represented as mean ± SEM from three independent experiments, each in triplicate. Source data are provided as a Source Data file