Abstract
Background and Aims:
Continuous wound infiltration of local anaesthetics provide postoperative analgesia by peripheral nociceptors blockade.The placement of wound infiltration catheter in the optimal anatomical plane of surgical wound may play a significant role in reducing postoperative pain depends on the surgical procedure. We hypothesised that preperitoneal infusion of local anaesthetics will reduce the postoperative opioid consumption as compared to subcutaneous infusion following cesarean section.
Methods:
This was a randomised, double-blinded clinical trial. Fifty-two pregnant women who underwent lower segment caesarean section by Pfannensteil incision, under spinal anaesthesia, were randomised to group 'subcutaneous’ and group ’preperitoneal’. A wound infiltration catheter was placed in the subcutaneous or preperitoneal plane, depending on their randomisation at the end of the surgery. Bupivacaine of 0.25% at 5 mL/h was infused for the next 48 h. Pain was assessed using numerical rating scale at 1, 2, 3, 4, 5, 6, 12, 24, 36 and 48 h after surgery. Cumulative postoperative consumption and adverse effects of morphine and complications of the procedure were looked for.
Results:
Cumulative 48-h morphine consumption showed no statistical significance between the preperitoneal group (15.96 ± 7.69 mg) and subcutaneous group (21.26 ± 11.03 mg); P = 0.058. Pain score was comparable. Independent T-test and Mann–Whitney test were the statistical tests used for continuous and categorical data, respectively.
Conclusion:
Postoperative cumulative morphine consumption and pain scores are comparable when bupivacaine is infused continuously through wound infiltration catheter either in the preperitoneal or subcutaneous layer following Caesarean delivery.
Keywords: C-section, postoperative analgesia, wound catheter
INTRODUCTION
Postcaesarean pain is a major limiting factor in attaining the goal of early postoperative functional recovery, and it is often traditionally managed with opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) either alone or in combination. Continuous wound infiltration (CWI) of local anaesthetics has been shown to minimise pain intensity and reduce the requirement of opioids and subsequently their side effects. In Pfannensteil incision for lower abdominal surgeries, the subcutaneous or preperitoneal plane are the common planes studied for CWI. The placement of the catheter within the wound in relation to the anatomical layers of the abdomen is identified as an important determinant of analgesic efficacy. However, current literature provides conflicting views regarding the ideal plane for analgesia in postcaesarean cases.[1,2,3] We hypothesised that preperitoneal infiltration of local anaesthetics would provide better analgesia, and therefore would reduce the first 48-h postoperative morphine consumption when compared with infiltration into the subcutaneous plane.
METHODS
On receiving approval from the institute ethics committee (Reg. no. JIP/IEC/2015/22/773), registration was done with the Clinical Trials Registry of India (Reg. no. CTRI/2016/07/007122), and enrolment of patients was carried out. Written informed consent was obtained from all participants.
This was a prospective double-blinded, randomised clinical trial, carried out on 52 parturients who underwent elective lower segment caesarean section (LSCS) under spinal anaesthesia. We recruited pregnant women, age 18–35 years, who were gravida 1 or 2, at 36 to 42 completed weeks of gestation belonging to American Society of Anesthesiologists physical status class 2 and 3 and scheduled for caesarean delivery by Pfannensteil incision. Exclusion criteria included patients who developed spinal anaesthesia–related problems (failure to achieve adequate spinal level, breakthrough intraoperative pain requiring opioid supplementation), intraoperative surgical difficulties (difficulty in closing peritoneum, excessive bleeding, uterine atony or operative injuries and placement of drain at wound site), stillbirth or newborns requiring intensive care unit care, history of allergy to local anaesthetics or NSAIDs, presence of any ongoing infection or sepsis and patients refusing spinal anaesthesia.
At the preanaesthetic visit, the procedure and purpose of the study were explained along with instructions regarding patient-controlled analgesia (PCA) device and numerical rating scale (NRS) for pain (0–10).
All parturients were premedicated with 20 mg oral famotidine and 10 mg oral metoclopramide on the day of the surgery. Electrocardiogram, noninvasive blood pressure and pulse oximetry were attached and monitored throughout the course of surgery. Peripheral intravenous (IV) access was secured and preloaded with 500 mL of Ringer's lactate solution. Spinal anaesthesia was instituted according to standard departmental protocol. Caesarean delivery was performed through a Pfannensteil incision. Eligible patients were randomised into 'subcutaneous group’ or ’preperitoneal group’ using computer-generated random number table. Group allocation was concealed in sealed opaque envelopes that were opened just before closure of the abdominal fascia and revealed to the operating surgeon by an independent researcher. The patient, investigator collecting the data and the anaesthesiologist attending the patient were blinded to the group allocation. In the subcutaneous group, after peritoneal closure, the rectus muscle and fascia were closed, and a 19G, 700-mm wound infiltration catheter (PAJUNK® InfiltraLong, Medizintechnologie, Germany) was placed over it along the length of the wound in the subcutaneous plane and skin was closed subsequently. In the preperitoneal group, after peritoneal closure the wound infiltration catheter was placed along the length of the wound so as to lie in the preperitoneal plane. The rectus fascia was then closed over it, followed by the skin. The catheters were secured at a point 1–2 cm beyond one end of the incision and provided with a separate sterile dressing. The patients were all blinded as to which technique they had received. Postoperatively in the recovery room, both groups of subjects received a 10-mL bolus of 0.25% bupivacaine through the catheter. Subsequently, the catheters were connected to elastomeric pumps (Easyfuser ambulatory pump, 275 mL; Nipro Australia, St Leonards NSW 2065, Australia) set to deliver 0.25% bupivacaine at 5 mL/h for the next 48 h. A single dose of IV paracetamol 1 g was given to all patients at the end of the surgery.
In addition, the patients were provided with i.v. morphine as PCA (Fresenius Master PCA pump, Fresenius Medical Care, North America) through a separate IV cannula. The PCA settings were 1 mg bolus, 7-m lockout interval and a maximum dose of 20 mg in 4 h. PONV would be treated with i.v. ondansetron. Antibiotics, IV fluids and uterotonic agents were provided as per obstetric department protocol. All patients who showed NRS of more than 6/10 received injection paracetamol 1 g IV as a rescue medication. Patients’ data were collected every hour for the first 6 h, subsequently at the next 6th hour followed by every 12th hour till 48 h of postoperative period. At the end of 48 h, the catheters were removed under aseptic conditions. Pain was assessed both at rest and on movement (sitting upright, right and left lateral position) at these hours.
The primary objective was to compare the morphine consumption in the first 48 h of postoperative period, after caesarean section, in subjects receiving CWI in the subcutaneous plane, against those receiving CWI in the preperitoneal plane. The secondary objectives were to compare the postoperative outcome in the subjects with respect to postoperative nausea and vomiting (PONV), pain at rest and at movement, adverse effects of opioids observed, early mobilisation and return of bowel movements and to assess method or catheter-related complications such as local site bleeding, wound discharge and infection.
Based on a previous study by Rackelboom et al.,[4] the average consumption of IV morphine in the first 48 h postoperative period after caesarean section was 22 mg, with a standard deviation of 8 mg. A sample size of 25 in each group (two groups) was needed to detect a clinically significant difference of 30% reduction in morphine consumption between the groups. The sample size was calculated using an alpha error of 0.05 and a beta error of 0.2. Consecutive sampling was done to recruit the patients.
The statistical software SPSS 18.0 and R environment ver. 3.2.2 were used for the analysis of the data. The results on continuous measurements are presented as mean ± standard deviation (SD) and the results on categorical measurements are presented in number (%). A P value less than 0.05 was considered as statistically significant.
Student's t-test (two-tailed, independent) was used to assess the significance of study parameters on continuous scale between two groups, such as cumulative morphine consumption, duration of hospital stay, NRS scores, time interval till first rescue analgesic and return of bowel movement.
Chi-square or Fisher's exact test was used to find the significance of study parameters with dichotomous data, such as PONV, pruritus and sedation.
RESULTS
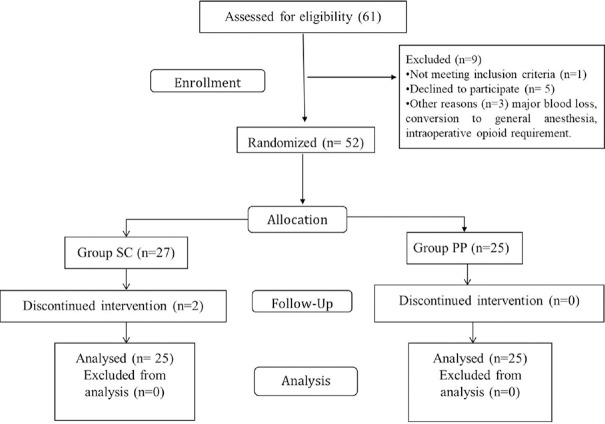
Sixty-one patients were assessed for eligibility, of which nine were excluded [Figure 1]. Demographic data between the groups were comparable [Table 1].
Figure 1.
Consort flow diagram
Table 1.
Comparison of demographic data between the subcutaneous and preperitoneal group.
| Data variable | Subcutaneous group (n=27) | Preperitoneal group (n=25) | p |
|---|---|---|---|
| Age (years) | 24.92±2.83 | 26.24±4.37 | 0.211 |
| BMI (kg/m2) | 24.66±4.86 | 26.33±2.45 | 0.132 |
| Duration of Surgery (min) | 110.60±26.19 | 102.40±21.94 | 0.236 |
| Indication for LSCS | |||
| Breech presentation | 2 (7.40%) | 6 (24%) | 0.247 |
| Contracted pelvis | 1 (3.7%) | 0 (0%) | |
| Previous caesarean section | 24 (88.8%) | 19 (76%) | |
| Gravida distribution | |||
| Gravida 1 | 4 (14.81%) | 7 (28%) | 0.306 |
| Gravida 2 | 23 (85.19%) | 18 (72%) |
Age, BMI and duration of surgery are expressed as mean (SD); indications for LSCS and gravida distribution expressed as number (proportion). BMI-Body mass index; LSCS-Lower section caesarean section
The cumulative morphine consumption in the two groups at 48 h after surgery was 21.26 ± 11.03 mg in the subcutaneous group and 15.96 ± 7.69 mg in the ’preperitoneal’ group, P = 0.058. The average postoperative morphine consumption at various time intervals between the groups is depicted in Figure 2, and the difference between the groups was not statistically significant.
Figure 2.
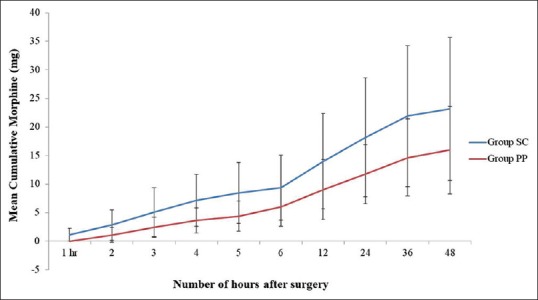
Cumulative post operative morphine consumption over 48 hours in subcutaneous group and preperitoneal group. Data expressed as mean (SD)
The first PCA use in the subcutaneous group was at 115.20 ± 129.87 min (mean with SD) after surgery, and in preperitoneal group it was at 114.0 ± 249.33 min, P = 0.96.
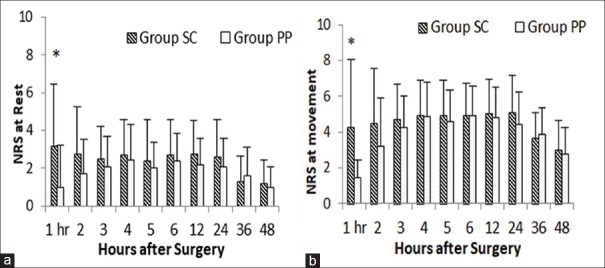
Analgesic efficacy was assessed at rest and movement by NRS score over the period of analysis. There was a significant difference between the two groups only in the first postoperative hour, both at rest and with movement [Tables 2, 3 and Figure 3].
Table 2.
Comparison of NRS for pain at rest, between the study groups
| Hours after surgery | NRS score | P | |
|---|---|---|---|
| Group SC | Group PP | ||
| 1 | 3.20±3.27 | 0.96±2.24 | 0.007* |
| 2 | 2.80±2.48 | 1.72±1.79 | 0.084 |
| 3 | 2.52±1.71 | 2.08±1.63 | 0.357 |
| 4 | 2.72±1.86 | 2.44±1.87 | 0.598 |
| 5 | 2.40±2.16 | 2.00±1.38 | 0.440 |
| 6 | 2.72±1.88 | 2.40±1.44 | 0.503 |
| 12 | 2.76±1.76 | 2.20±1.41 | 0.221 |
| 24 | 2.64±1.96 | 2.08±1.53 | 0.264 |
| 36 | 1.32±1.35 | 1.60±1.50 | 0.491 |
| 48 | 1.20±1.22 | 0.96±1.10 | 0.469 |
NRS-Numerical rating scale; SC-Subcutaneous; PP-Preperitoneal; NRS scores expressed as mean±SD.*P<0.05–significant
Table 3.
Comparison of NRS on movement, in two groups of patients studied
| Hours after surgery | NRS score | P | |
|---|---|---|---|
| Group SC | Group PP | ||
| 1 | 4.24±3.78 | 1.44±2.93 | 0.005* |
| 2 | 4.48±3.06 | 3.20±2.69 | 0.123 |
| 3 | 4.68±1.99 | 4.24±1.79 | 0.415 |
| 4 | 4.92±1.98 | 4.84±1.95 | 0.886 |
| 5 | 4.92±1.96 | 4.56±1.76 | 0.497 |
| 6 | 4.92±1.80 | 4.92±1.66 | 1.000 |
| 12 | 5.00±1.96 | 4.80±1.73 | 0.704 |
| 24 | 5.08±2.06 | 4.40±1.83 | 0.223 |
| 36 | 3.64±1.41 | 3.84±1.49 | 0.628 |
| 48 | 2.96±1.65 | 2.76±1.48 | 0.653 |
NRS-Numerical rating scale; SC-Subcutaneous; PP-Preperitoneal; NRS scores expressed as mean±SD *P<0.05–significant
Figure 3.
Post operative pain assessed by numerical rating scale for pain at rest (a) and movement (b) assessed at 1,2,3,4,5,6,12,24, 36 and 48 hours after surgery, in subcutaneous group vs. preperitoneal group. Data expressed as mean (SD)
There was no incidence of serious adverse events, systemic local anaesthetic toxicity, postoperative nausea or vomiting or wound site discharge or skin changes on removal of the catheter. Return of bowel movements was assessed by passage of first flatus. About 60% of patients in the subcutaneous group reported it within 48 h after surgery; while in preperitoneal group, 72% recorded the same and was found statistically not significant.
The average duration of hospital stay was 5.52 ± 0.87 days in the subcutaneous group and 5.32 ± 0.69 days in the preperitoneal group, P = 0.37.
Eight (32%) patients in the subcutaneous group expressed satisfactory analgesia, whereas 13 (52%) expressed the same in the preperitoneal group, P = 0.152. This was not significant.
Two patients in the subcutaneous group developed abdominal distension with generalised tightness and pain at the end of 48 h, with no tenderness at the incision site. Recovery of bowel movements was also delayed in these patients to more than 48 h. Stool softeners were provided and symptomatic recovery was achieved. In two patients in the subcutaneous group at the end of 48 h, the distal part of the catheter was retained within the wound as it accidentally snapped while being removed leaving behind a substantial length of the catheter within the wound. The obstetrician removed it under local anaesthesia and aseptic cover by removing a couple of skin sutures of the incision. The whole length of the left back catheter was removed, incision resutured and further follow-up for abnormal wound discharge or infection was done, yielding uneventful results.
DISCUSSION
In this randomised, controlled, double-blinded study, we found that the difference in 48-h cumulative morphine consumption was statistically not significant between the preperitoneal and subcutaneous continuous infusion of local aesthetics through wound catheter. There was also no statistically significant difference in NRS score both at rest and with movement, except for the first postoperative hour.
Postoperative pain following lower segmental caesarean section, as in any abdominal surgeries, is due to the nociceptive receptors present at various layers, that is, the peritoneal layer which carries the major visceral pain fibres, musculofascial layer and the superficial layer of subcutaneous and skin which carries the somatic pain fibres. While there are various analgesic modalities used to address this issue, multimodal analgesic technique is often used to achieve satisfactory pain relief. One of the postoperative multimodal analgesic combinations used is CWI catheters with local anaesthetics along with IV nonsteroidal anti-inflammatory agents and opioids. Although the feasibility, efficacy and safety of local anaesthetic infusion through wound infiltration catheter for postcaesarean analgesia were proven well by previous studies, the optimal site of placement is still under debate.[5]
A metanalysis analysed 32 heterogeneous studies on wound catheters and concluded that only a subgroup of gynaecologic and obstetric surgeries showed a significant reduction in VAS scores along with a modest reduction in morphine consumption when wound catheters were used primarily as a postoperative analgesic modality.[5] In that study, they found that subfascial or preperitoneal placement of catheters resulted in better analgesia; however, the surgical population attributed to this finding is heterogeneous, comprising different forms of surgeries and sites, hence calling for better designed studies.
In caesarean surgical population, continuous local anaesthetic infiltration in the subcutaneous plane resulted in significant reduction in postoperative morphine consumption when compared with saline placebo.[6] Mecklem et al., on the other hand, placed the catheter in the preperitoneal plane and showed similar results with the same drug formulation.[7] These conflicting results forced other authors to study whether the plane of placement of wound catheter in the abdominal wall will affect the efficacy of this mode of analgesia in terms of its opioid spring effect.
A randomised double blinded trial[4] in caesarean patients studied the subcutaneous and preperitoneal infiltration of mixture of local anaesthetics and NSAIDs for analgesic efficacy and they found that the 48-h cumulative morphine use was 57% less in the preperitoneal group when compared with subcutaneous group. We decided to study the efficacy of wound infusion local aesthetics alone without addition of NSAIDS in relation to its site of placement and hypothesised that preperitoneal wound infusion would result in better opioid-sparing effect when compared with subcutaneous infusion. Except for the use of intrathecal morphine, local anaesthetic and NASID mixture, we formulated a study similar to this trial. They had postulated that combination of local anaesthetics and NSAIDs would have resulted in better anti-inflammatory effect when administered in the preperitoneal layer and better visceral analgesia because the infused mixture spreads near to the peritoneal injury as shown by magnetic resonance imaging.
Infusion of local anaesthetics alone above the abdominal fascia following abdominal hysterectomy resulted in reduced rescue opioid requirement in a study by Hafizoglu et al. They had explained that somatic origin pain would have been the major contributor to postoperative pain following abdominal hysterectomy.[8]
Compared with these two studies, our results showed that in caesarean surgical population the route of administration of local anaesthetic infusion is not a major determinant in the reduction of postoperative opioid consumption. Rather, the nature of drug combination infused like addition of NSAID in the local anaesthetic mixture and surgical setting play a major role in the opioid-sparing effect. Furthermore, our study results explain the equal contribution of visceral and somatic pain to postoperative discomfort following caesarean section. Previous study using P wound infiltration on the effective method for pain management after colorectal surgery reported no technical difficulty in their study when catheters were place in the preperitoneal plane, owing to their expertise in this procedure.[9] In our institute, this modality is a fairly new venture and surgical dexterity in placing, fixing the catheter and its removal, is at its learning stage. With wider use of this method, complications are expected to decline.
Although we did not study the postoperative morphine consumption using saline placebo, other studies have well proved the analgesic superiority of wound infiltration catheter.[6,7] Therefore, with our results, we infer that for an effective postoperative analgesia by wound infiltration catheters following lower segmental caesarean section the nociceptive receptors present in all three levels, that is, visceral, musculofascial and cutaneous tissue have to be taken care. As reported by previous studies, the mean first 48-h postoperative morphine consumption ranges between 50 and 70 mg after spinal anaesthesia with intrathecal morphine.[4] As evident from our study, targeting any one of these layers by local anaesthetic infusion through wound infusion catheter may be an effective way to reduce this cumulative morphine consumption further and thereby minimise opioid-related side effects.
There are few limitations in the study. First, addition of regular doses of parenteral NSAIDs to multimodal analgesia would have reduced the cumulative morphine consumption further thereby improved the postoperative pain scores and opioid-related side effects. Second, radiological imaging of drug spread could have yielded further insight into the possible site of drug delivery with regard to preperitoneal and subcutaneous infusion of local anaesthetics.
CONCLUSION
Postoperative cumulative morphine consumption and pain scores are comparable when bupivacaine is infused continuously through wound infiltration catheter either in the preperitoneal or subcutaneous layer following Caesarean delivery.
Financial support and sponsorship
Funding was provided by Institute intramural research fund (JIP/Res/Intra-MD-MS/phs1/01/2016-17) for postgraduate dissertation.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.O’Neill P, Duarte F, Ribeiro I, Centeno MJ, Moreira J. Ropivacaine continuous wound infusion versus epidural morphine for postoperative analgesia after cesarean delivery: A randomized controlled trial. Anesth Analg. 2012;114:179–85. doi: 10.1213/ANE.0b013e3182368e87. [DOI] [PubMed] [Google Scholar]
- 2.de Almeida MC, de Figueiredo Locks G, Brunharo GM, Kauling AL. Postoperative analgesia: Comparing continuous epidural catheter infusion of local anesthetic and opioid and continuous wound catheter infusion of local anesthetic. Braz J Anesthesiol. 2011;61:293–303. doi: 10.1016/S0034-7094(11)70035-6. [DOI] [PubMed] [Google Scholar]
- 3.Beaussier M, El’Ayoubi H, Schiffer E, Rollin M, Parc Y, Mazoit J-X, et al. Continuous preperitoneal infusion of ropivacaine provides effective analgesia and accelerates recovery after colorectal surgery: A randomized, double-blind, placebo-controlled study. J Am Soc Anesthesiol. 2007;107:461–8. doi: 10.1097/01.anes.0000278903.91986.19. [DOI] [PubMed] [Google Scholar]
- 4.Rackelboom T, Le Strat S, Silvera S, Schmitz T, Bassot A, Goffinet F, et al. Improving continuous wound infusion effectiveness for postoperative analgesia after cesarean delivery: A randomized controlled trial. Obstet Gynecol. 2010;116:893–900. doi: 10.1097/AOG.0b013e3181f38ac6. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Favaios S, Perniola A, Magnuson A, Berggren L. A meta-analysis of the efficacy of wound catheters for post-operative pain management. Acta Anaesthesiol Scand. 2011;55:785–96. doi: 10.1111/j.1399-6576.2011.02463.x. [DOI] [PubMed] [Google Scholar]
- 6.Givens VA, Lipscomb GH, Meyer NL. A randomized trial of postoperative wound irrigation with local anesthetic for pain after cesarean delivery. Am J Obstet Gynecol. 2002;186:1188–91. doi: 10.1067/mob.2002.122984. [DOI] [PubMed] [Google Scholar]
- 7.Mecklem DW, Humphrey MD, Hicks RW. Efficacy of bupivacaine delivered by wound catheter for post-Caesarean section analgesia. Aust N Z J Obstet Gynaecol. 1995;35:416–21. doi: 10.1111/j.1479-828x.1995.tb02156.x. [DOI] [PubMed] [Google Scholar]
- 8.Hafizoglu MC, Katircioglu K, Ozkalkanli MY, Savaci S. Bupivacaine infusion above or below the fascia for postoperative pain treatment after abdominal hysterectomy. Anesth Analg. 2008;107:2068–72. doi: 10.1213/ane.0b013e318187ed23. [DOI] [PubMed] [Google Scholar]
- 9.Ozer A, Yilmazlar A, Oztürk E, Yılmazlar T. Preperitoneal catheter analgesia is an effective method for pain management after colorectal surgery: The results of 100 consecutive patients. Local Reg Anesth. 2014;7:53–7. doi: 10.2147/LRA.S71476. [DOI] [PMC free article] [PubMed] [Google Scholar]