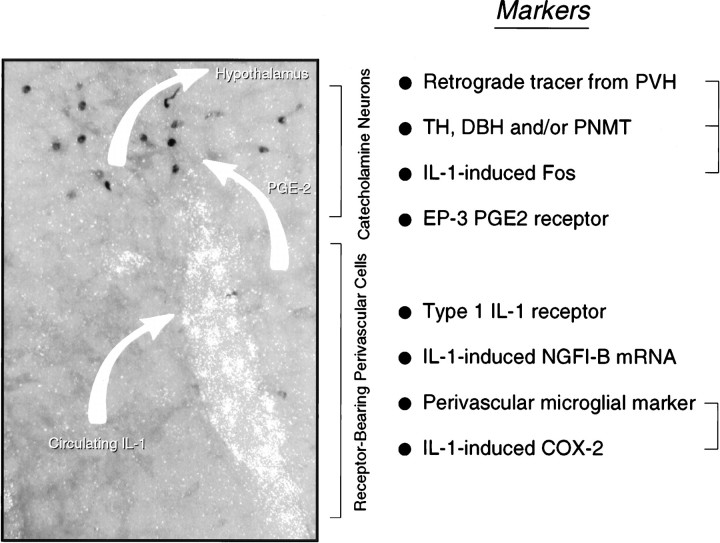
Fig. 8.
Possible mechanism for intravenous IL-1-mediated stimulation of central HPA control systems. A polarized epifluorescence illumination image of a section through the rostral ventrolateral medulla shows combined hybridization histochemical localization of perivascular cells displaying IL-1R1 mRNA and immunoperoxidase detection of nuclear Fos-ir in the region of the C1 catecholamine cell group. We suggest that circulating IL-1 binds its cognate receptor on perivascular cells in the region, inducing them to synthesize PGE2, which in turn diffuses through the extracellular space to (directly or indirectly) stimulate nearby aminergic neurons and, consequently, CRF-expressing targets of their axonal projections in the endocrine hypothalamus. Listed at the right are markers of potentially relevant components of this signaling cascade that have been localized in the requisite regions, either under basal conditions or in response to intravenous IL-1 or endotoxin. Markers that have been co-localized to date are bracketed. It remains to be determined how the others are distributed with respect to the key perivascular (i.e., IL-1R1-expressing) and neuronal (IL-1-sensitive, hypothalamically projecting, and catecholaminergic) cell types.