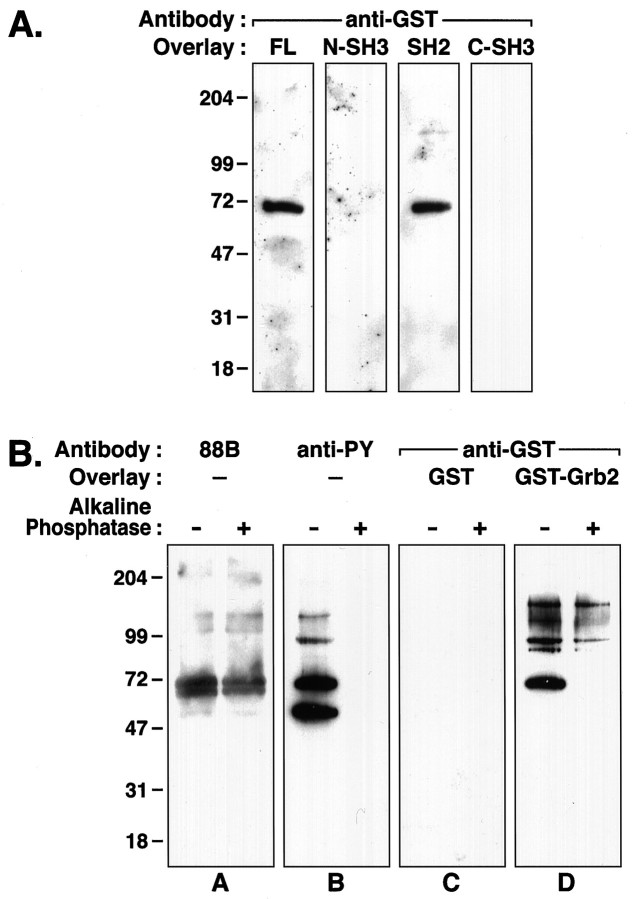
Fig. 2.
Grb2-δ subunit binding is mediated by an SH2–phosphotyrosine interaction. A, IsolatedTorpedo AChR subunits were resolved by SDS-PAGE, transferred to nitrocellulose, and subjected to protein overlay analysis. Full-length GST–Grb2 (FL) binds to the δ subunit, as shown in Figure 1. No binding is observed with either SH3 domain fusion protein of Grb2 (N-SH3 and C-SH3). Fusion proteins containing the SH2 domain (SH2), however, bind to the δ subunit. B, Equivalent amounts ofTorpedo membranes (5 μg) treated with (right lane) and without (left lane) alkaline phosphatase were subjected to SDS-PAGE and transferred to nitrocellulose for protein overlay analysis. Panel A, Immunoblot with mAb 88B shows that approximately equal amounts of δ subunit protein were loaded for each condition.Panel B, Parallel immunoblots, using a cocktail of anti-phosphotyrosine (anti-PY) antibodies, 4G10 and PY20, indicate an absence of immunoreactivity in the phosphatase-treated membranes. Panel C, Protein overlays with control GST show no binding. Panel D, Binding of full-length GST–Grb2 to the δ subunit is abolished by phosphatase treatment. Binding to the 90 and 150 kDa bands is retained.