Fig. 3.
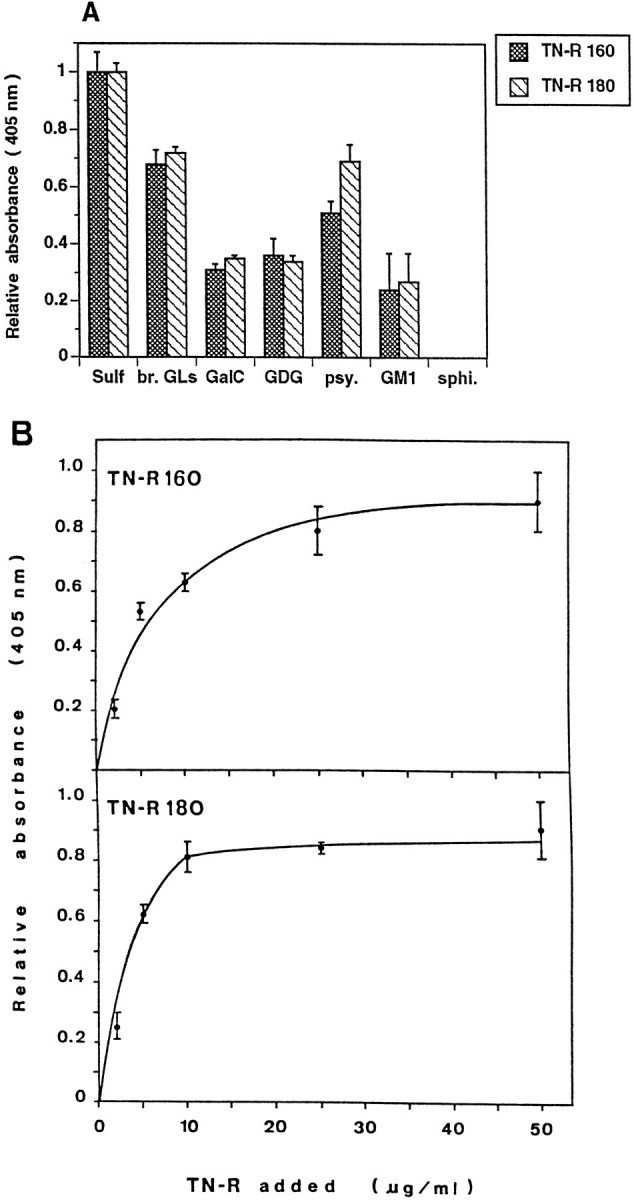
Binding of TN-R 160 and TN-R 180 to polar GLs, as determined by solid phase ligand-binding assay. The fraction of brain polar GLs (br. GLs, 2 μg/ml in PBS) orSulf, GalC, galactosyl diglyceride (GDG), psychosine (psy.),GM1, and sphingosine (sphi., all at 1 μg/ml in PBS) were coated into wells of microtiter plates, and the binding of biotinylated TN-R was determined after 2 hr of incubation at 37°C (A). B, Immobilized Sulf (at 0.5 μg/ml in 100% ethanol) was incubated with increasing amounts of TN-R 160 or TN-R 180 (2–50 μg/ml). Bound protein was detected by a subsequent incubation with polyclonal antibodies to TN-R (2 hr at 37°C), and antibody binding was visualized by using HRP-conjugated goat anti-rabbit IgG and ABTS (2,2-azino-di-[3-ethylbenzthiazoline sulfonate(6)]; Boehringer Mannheim). Background binding to BSA was subtracted from the values obtained for the binding to different GLs. The maximal absorbance at 405 nm for the binding of each TN-R isoform to Sulf was set as 1.0. Values represent the mean ± SD of three (A) or two (B) experiments performed in triplicate.
