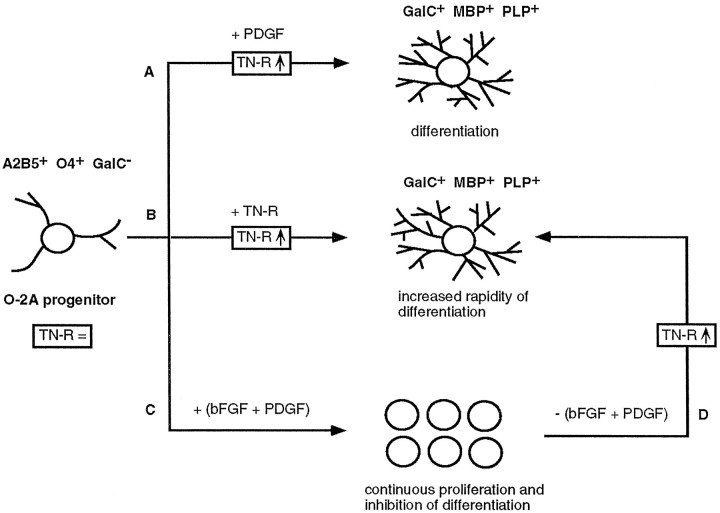
Fig. 8.
Hypothetical model on the molecular control of O-2A progenitor cell (A2B5+O4+GalC−) differentiation into mature OLs—a recapitulation of the data in the literature from the viewpoint of TN-R. O-2A progenitors express relatively low amounts of TN-R (TN-R =). PDGF drives the clock that times O-2A cell differentiation into GalC+ OLs (A). The effect of PDGF likely is attributable to the upregulation of TN-R expression by O-2A cells (TN-R ↑), which, in turn, stimulates their terminal differentiation into myelin-forming cells (GalC+MBP+PLP+), because TN-R alone upregulates its own expression and induces the terminal differentiation of O-2A progenitors in the absence of PDGF (B). The simultaneous action of bFGF and PDGF leads to continuous cell proliferation and inhibition of differentiation by a mechanism that interferes with the differentiation-inducing activity of PDGF (C). On removal of the two growth factors (D), a critical threshold of intrinsic TN-R expression (TN-R ↑) then would be a prerequisite for the ensuing rapid differentiation of O-2A cells, similar to B. This model does not discriminate whether the upregulation of TN-R expression occurs in the presence and/or after the removal of the mitogens. TN-R thus may represent an intrinsic differentiation factor, the expression of which is subject to regulation by growth factors.