Fig. 2.
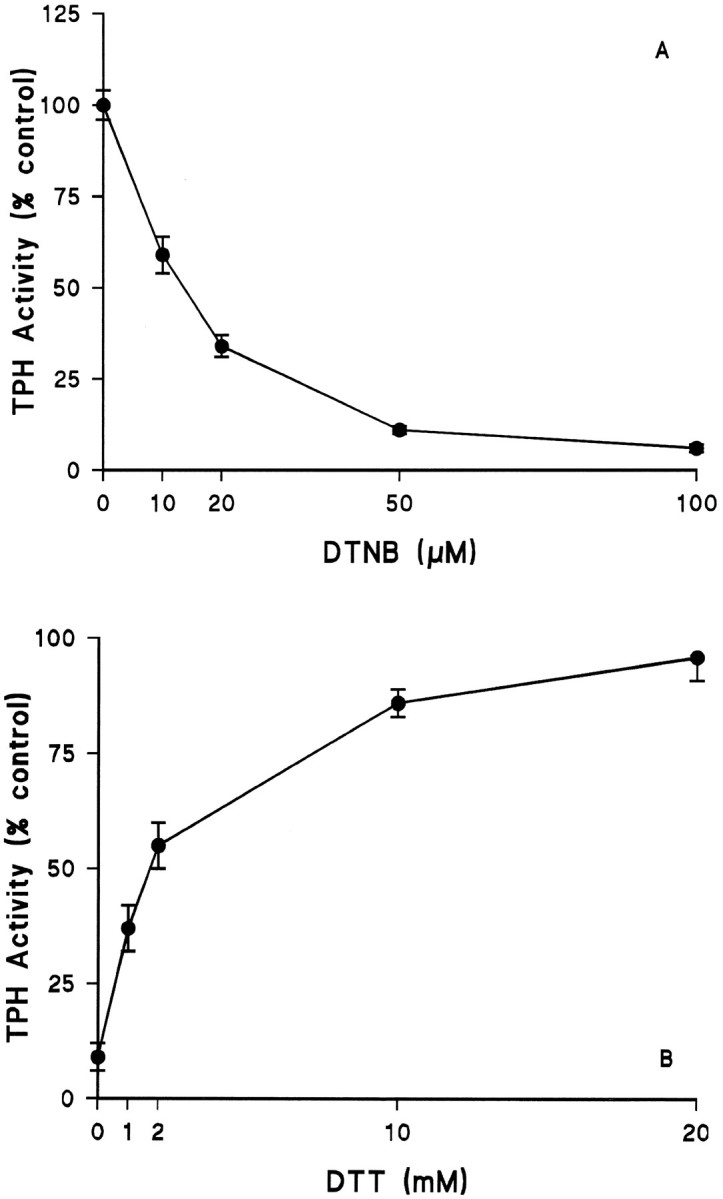
Effects of the disulfide DTNB on TPH. TPH–CC protein was prepared and adsorbed to GSH-affinity beads as described in Materials and Methods. A, The enzyme was exposed to varying concentrations of DTNB (without DTT) at 30°C for 15 min followed by three washes with 50 mm Tris-HCl, pH 7.5, at 4°C. Residual TPH activity was determined in the presence of 100 μm ferrous ammonium sulfate without DTT.B, TPH–CC was first exposed to 50 μm DTNB as described in Figure 2A and then GSH-affinity beads were washed free of DTNB. The enzyme was next exposed to varying concentrations of DTT at 4°C for 30 min. After DTT treatment, beads were washed as before, and residual TPH activity was determined in assays that omitted DTT. The results in both A andB are expressed as % control TPH activity (51.5 nmol of 5-HTP · min−1 · mg−1) and are the mean ± SEM of four independent experiments performed in duplicate. The effects of DTNB in A and that of DTT in B were both statistically significant by ANOVA;p < 0.001 for each.
