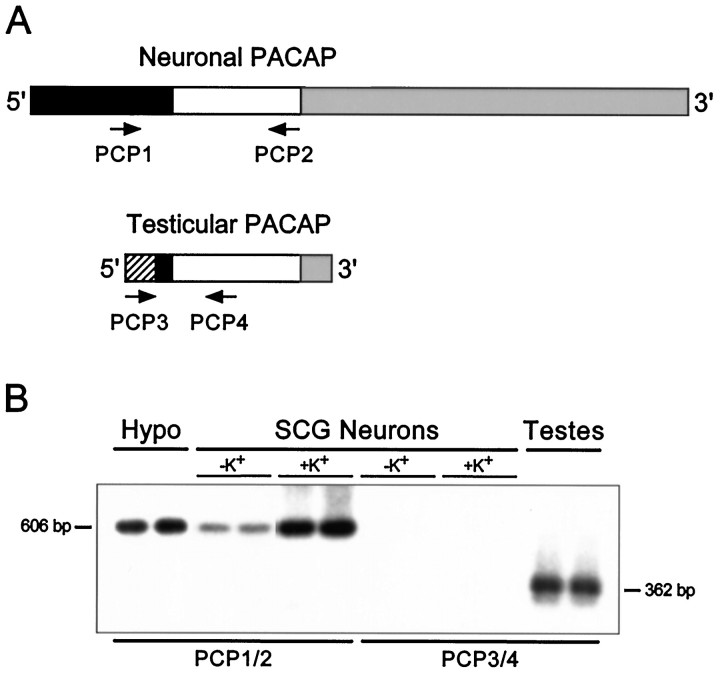
Fig. 9.
Reverse transcription-PCR and gene-specific hybridization reveal that the smaller pro-PACAP transcript induced in SCG neurons is not the testicular form of the message.A, Schematic representation of the neuronal and testicular pro-PACAP transcripts demonstrating the positions of the transcript-specific oligonucleotide primer pairs; the neuronal pro-PACAP transcript-specific primers are PCP1 and PCP2 and the testicular pro-PACAP transcript-specific primers are PCP3 and PCP4 (Table 1). White, coding region; black, 5′ untranslated region; gray, 3′-untranslated region;hatched, testes-specific 5′-untranslated region.B, Total RNA from individual SCG neuronal cultures incubated in medium containing 40 mm NaCl (−K+) or 40 mm KCl (+K+) was reverse-transcribed, and the cDNA was amplified using the neuronal transcript-specific primers (PCP1 and PCP2) or the testicular transcript-specific primers (PCP3 andPCP4). Total hypothalamic (Hypo) and testicular (Testes) RNA were reverse-transcribed and amplified with the neuronal and testicular primer pairs, respectively. The amplified products were fractionated, blotted, and hybridized with a radiolabeled synthetic internal oligonucleotide probe located within the pro-PACAP coding region that is identical for the neuronal and testicular transcripts; the membranes were apposed to film for 150 min (SCG neurons ± K+, both primer pairs), 70 min (hypothalamus), or 40 min (testes). The predicted product sizes are 606 bp for the neuronal transcript-specific primers and 362 bp for the testicular transcript-specific primers. Representative samples are shown; identical results were obtained from two independent culture preparations consisting of four to six individual sympathetic culture wells for each treatment.