Fig. 2.
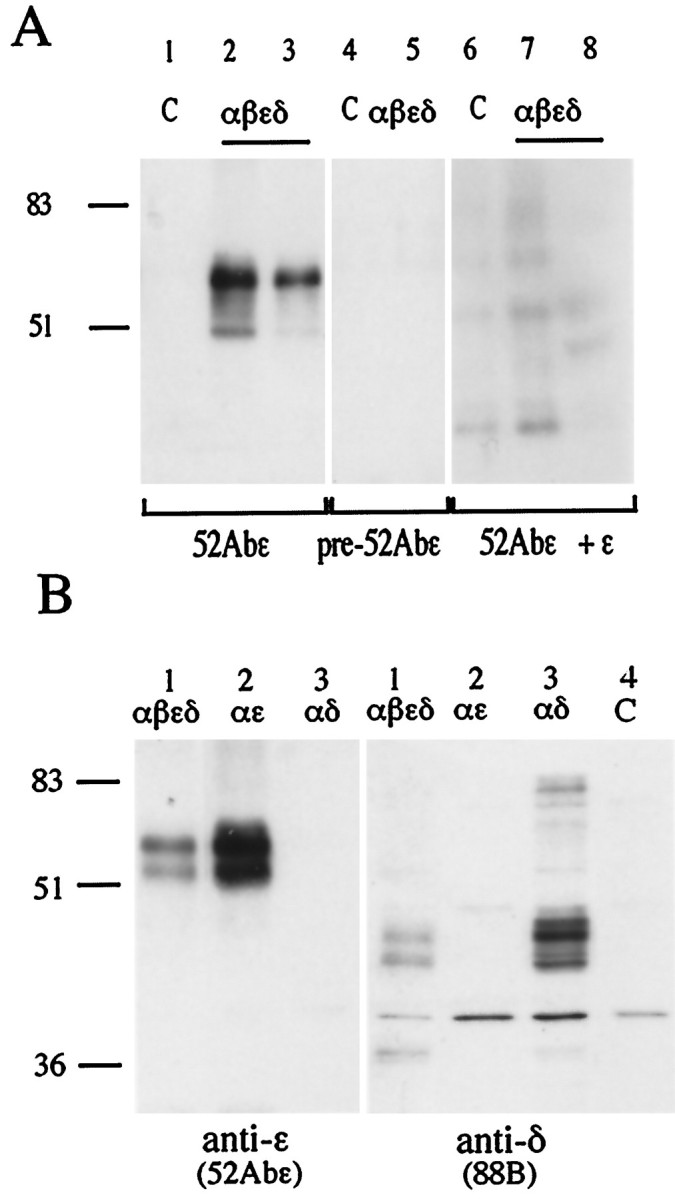
The anti-ε 52Abε polyclonal antibody specifically recognizes the ε AChR subunit on Western blots.A, HEK 293 kidney fibroblast cells were transfected with cDNAs coding for the αβεδ subunits (2:1:1:1) of rat AChR (lanes 2, 3, 5, 7, 8) or left as untreated controls (lanes 1, 4, 6). The 52Abε antibody recognized a major band at molecular weight ∼60 kDa in the ε-containing AChR-transfected cells (lanes 2, 3) but not in the control nontransfected cells (lane 1). No bands were seen with preimmune serum (lanes 4, 5) or after preabsorption of the 52Abε antibody with the ε peptide (lanes 6–8). Numbers on theleft correspond to molecular mass standards (in kilodaltons). B, Samples obtained from HEK 293 cells transfected with αβεδ (2:1:1:1), αε (2:1), or αδ (2:1) cDNAs were incubated with either the anti-ε subunit 52Abε antibody or the anti-γ/δ subunit mAb 88B. The 52Abε antibody recognized a major bands of 60 kDa in the cells transfected with αβεδ and αε but none in the cells transfected with αδ. mAb 88B specifically immunodecorated the lanes loaded with membrane preparations obtained from the cells transfected with αβεδ and αδ but not from cells transfected with αε or the nontransfected control. (C).
