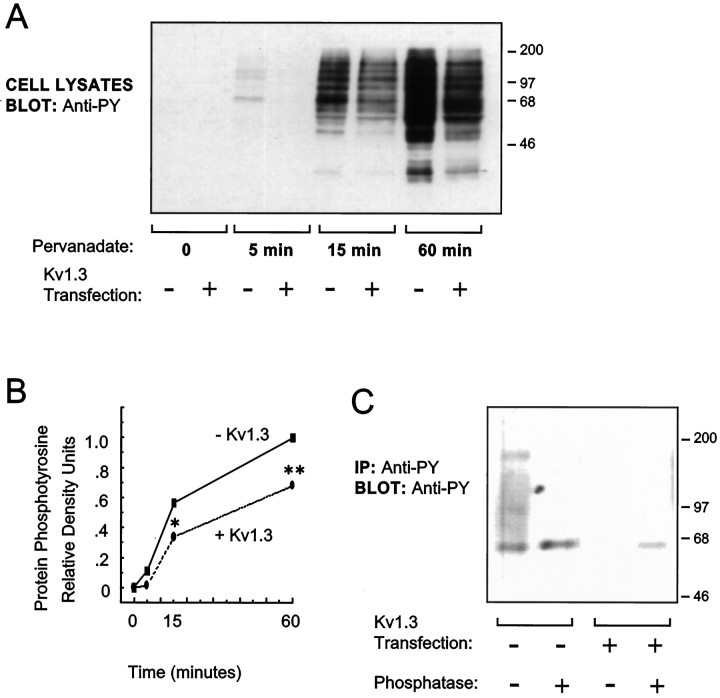
Fig. 1.
Kv1.3 expression decreases endogenous protein tyrosine phosphorylation in pervanadate-treated HEK 293 cells. Cells were transfected with cDNA for either control vector (−) or Kv1.3 (+). Two days after transfection, cells were treated with the membrane-permeant tyrosine phosphatase inhibitor pervanadate (250 μm; 0–60 min). A, Cell lysates were prepared, and the lysate proteins were separated by SDS-PAGE and electrotransferred to nitrocellulose. Immunoblots were probed with anti-phosphotyrosine antibody (anti-PY). Primary antibody binding was visualized by incubation with a horseradish peroxidase-conjugated secondary antibody and ECL and autoradiography.B, Protein phosphotyrosine signal was quantified in eachlane of the blot in A by densitometry, and values are expressed relative to that in the pervanadate-treatedlane, without Kv1.3 expression, at 60 min (n = 4; *p ≤ 0.05 and **p ≤ 0.01, Student’s t test).C, Tyrosine-phosphorylated proteins were immunoprecipitated (IP) using anti-phosphotyrosine antibody. Half of the IP samples were treated with alkaline phosphatase overnight (+). All IP samples were separated by SDS-PAGE and electrotransferred to nitrocellulose. Immunoblots (Blot) were probed with another anti-phosphotyrosine antibody. An artifactualband of apparent molecular weight slightly greater than the major tyrosine-phosphorylated band is present in the alkaline phosphatase-treated samples.