Fig. 4.
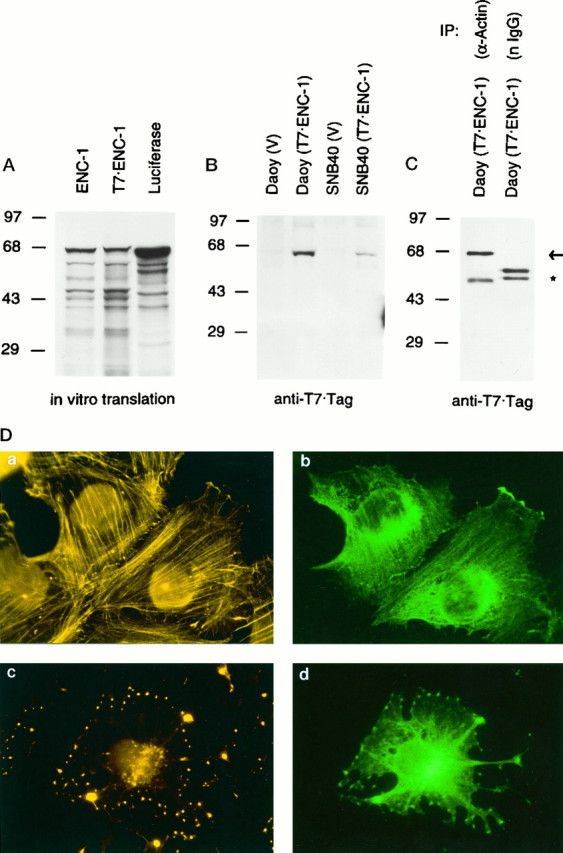
In vivo association between ENC-1 and actin, and cellular localization of ENC-1. A,In vitro synthesis of 35S-radiolabeled full-length ENC-1 from ENC-1 and T7-tagged ENC-1 expression vectors. Autoradiogram of SDS-PAGE resolved ENC-1 and T7-tagged ENC-1 proteins after synthesis by in vitro transcription–translation assays. Luciferase was used as a control. Positions of the molecular mass markers in kilodaltons are shown at left.B, Stable expression of T7-tagged ENC-1 in Daoy and SNB40 cell lines. Immunoblot analysis with T7tag monoclonal antibody was performed from stably transfected Daoy and SNB40 cell lines with T7-tagged ENC-1 expression vector and corresponding vector control. Positions of the molecular mass markers in kilodaltons are shown atleft. C, In vivoassociation between ENC-1 and actin. Lysates from Daoy cells stably transfected with T7-tagged ENC-1 were immunoprecipitated with a polyclonal antibody against actin or with normal rabbit Igs. Immunoprecipitates were then analyzed by immunoblotting with T7 tag monoclonal antibody. The T7-tagged ENC-1 (67 kDa) was present in the anti-actin immunoprecitate, but not in the control (arrow). Cross-reactivity with rabbit heavy-chain IgG is indicated by an asterisk. Normal rabbit Igs were used in an excess concentration in relation to the affinity-purified anti-actin antibody, which accounts for a stronger signal of the heavy-chain IgG in the control immunoprecipitate. Positions of the molecular mass markers in kilodaltons are shown at left.D, Co-localization of ENC-1 with the actin cytoskeleton. T7-tagged ENC-1 was stably expressed in Daoy cells and localized by immunofluorescence with a mouse T7tag monoclonal antibody followed by FITC-conjugated goat anti-mouse Ig (b,d). In the same cells, actin filaments were visualized with TRITC-labeled phalloidin (a,c). Cells fixed after a 30 min treatment with cytochalasin D to disrupt actin filaments were also examined (c, d). Photomicrographs were taken from the same field using filters for rhodamine (left) and fluorescein (right). Magnification 630×.
