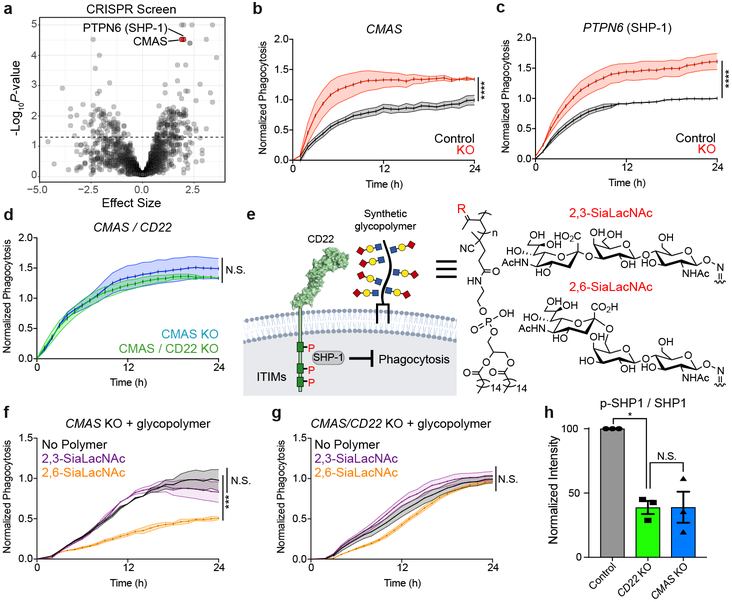
Figure 2. CD22 mediates the anti-phagocytic effect of α2-6-linked sialic acid.
a, Results from CRISPR-Cas9 screen targeting 2,015 drug targets, kinases, and phosphatases in BV2 cells (screen performed in technical duplicate; dashed line, P=0.05, two-sided t-test).
b, c, d, Phagocytosis of pH-sensitive fluorescent beads by control (black) vs CMAS KO (red) BV2 cells (b), control (black) vs PTPN6 KO (red) cells (c), and CMAS KO (blue) vs CMAS/CD22 double KO (green) cells (d) (n=3, ****P<0.00005, N.S. not significant, two-sided t-test; mean +/− s.e.m.).
e, Cell-surface glycan engineering using lipid tail-functionalized glycopolymers bearing sialic acid α2,3- or α2–6-linked to N-acetyllactosamine. ITIMs = immunoreceptor tyrosine-based inhibitory motifs, SHP-1 = Src homology region 2 domain-containing phosphatase-1.
f, g, Phagocytosis of pH-sensitive fluorescent beads by CMAS KO (f) or CMAS/CD22 double KO (g) BV2 cells coated with no polymer (black), α2,3-linked sialic acid (purple) or α2,6-linked sialic acid (orange) (n=3, ***P<0.0005, N.S. not significant, one-way ANOVA with Tukey’s correction; mean +/− s.e.m.).
h, Western blot quantification of ratio of active phosphorylated SHP1 (p-SHP1) to total SHP1 in control (gray), CD22 KO (green), and CMAS KO (blue) BV2 cells, normalized to a loading control protein (α-tubulin) (n=3, *P<0.05, N.S. not significant, one-way ANOVA with Tukey’s correction). For raw source image, see Supplementary Figure 1.
Data in b-h were replicated in at least 2 independent experiments.