Figure 1.
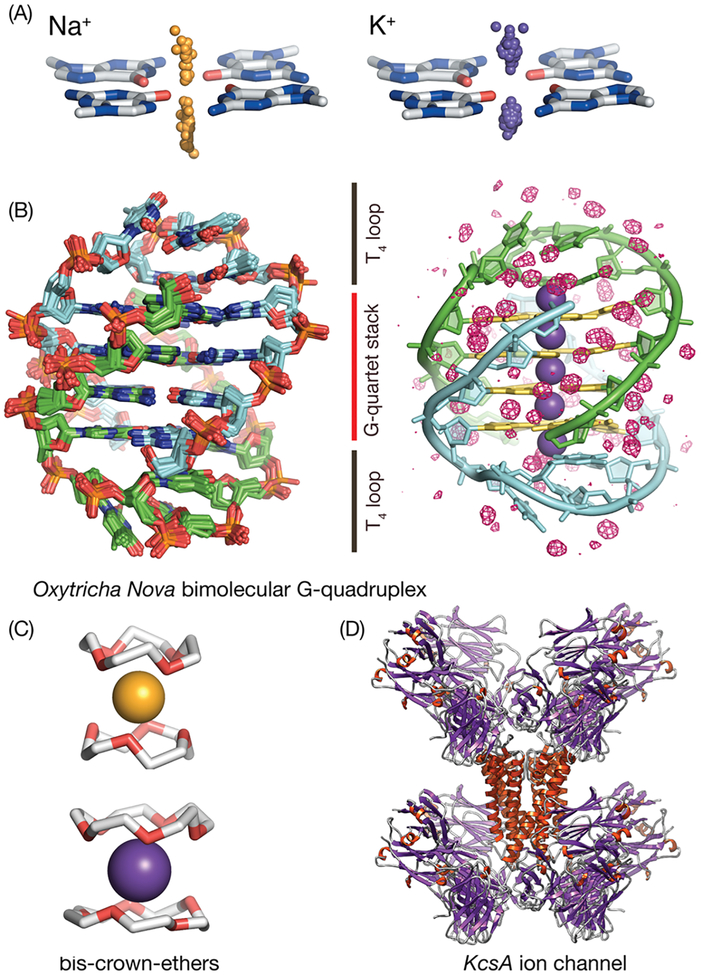
Nucleic acid quadruplexes form inner channels using stacked G-quartets within which Na+ and K+ cations are bound in a size-dependent manner. (A) K+ and Na+ adopt different binding patterns with respect to G-quartets, as shown by the distribution of Na+ cations (orange spheres) and K+ cations (right, violet spheres) with respect to a reference G-quartet obtained from all existing GQ crystal structures available in the PDB. (B) Oxy-GQ maintains the same fold in crystal and in solution and in the presence of Na+ or K+.1,38 (B, left) Ensemble of superimposed crystallized structures of Oxy-GQ used in this work. The maximum heavy atom RMSD between its members is 0.7 Å. The two strands of Oxy-GQ are shown in green and light blue, respectively. (B, right) Solvation structure of Oxy-GQ that has an internal channel with five cation-binding sites (shown as violet spheres); K+ cations bind near the midpoint between G-quartets, while Na+ cations bind along the same channel but their positions are shifted near the G-quartets, in consensus with the trend observed for all G-quartets in the PDB. The guanine rings that form the quadruplex are highlighted in yellow. The crystallographic consensus solvation water map (isosurface red mesh) reveals tightly bound waters deeply buried within the T-loops that make direct contact with channel-bound cations and along the grooves. (C, D) The manner in which GQs bind cations bears a high degree of similarity to that of crown-ethers and ion channels. This work uses crystallographic structural and thermodynamic data on cation binding to crown ethers and ion channels to improve the underlying force-field used together with 3D-RISM and to enable quantitative predictions on the population of cations inside the GQ channel as a function of salt concentration.