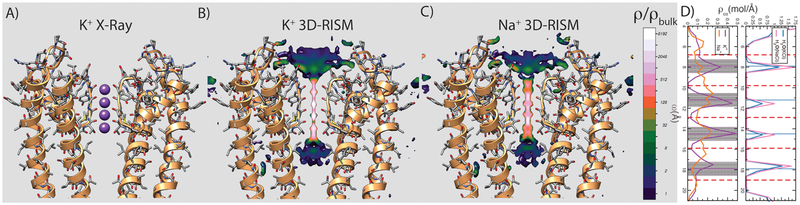
Figure 2.
Validation of the calibrated force field using crystallographic and thermodynamic data for cation and water binding in the selectivity filter of the KcsA ion channel. (A) Overlapping crystallographic K+- and water-binding loci in the selectivity filter of the KcsA ion channel are marked using violet spheres. Crystallographic data suggest that K+ and water molecules have approximately 50% occupancy in each locus in a background of 200 mM monovalent salt. For simplicity only two units out of four of the ion channel are shown (orange ribbons). (B) Tomographic slice along the selectivity filter through the 3D-RISM K+ density and (C) Na+ density, respectively. (D) (Left) Worm projections along the selectivity filter for K+ (violet solid line) and Na+ (orange solid line) and (right) water component of the NaCl solution (solid pink line) and KCl solution (blue solid line). The positions of the five coordinating rims of the selectivity filter are shown by red dashed lines. Crystallographic positions of K+ and water in the selectivity filter are marked using horizontal violet and light-blue lines, respectively. Transparent violet bars mark the width of K+ distribution inside (obtained from the B-factor) for each binding locus. Using the calibrated force field the average occupancy of each binding locus was estimated at approximately 46% for cations and 50% for water.