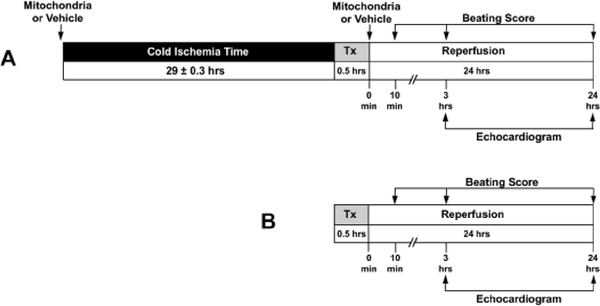
Fig. 1.
Experimental protocol. (A) C57BL/6J male mice (n = 17, 7–9 weeks) were used for all experimental groups. Donor hearts received 1 × 108 mitochondria in 0.5 mL respiration buffer (Mitochondria, n = 8) or 0.5 mL respiration buffer (Vehicle, n = 9) delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 40 G needle, 10 minutes before organ harvest. Heart grafts were then preserved in normal saline solution containing 1% Penicillin and 1% Streptomycin at 4 °C for 29 ± 0.3 hours prior to heterotopic heart transplantation. Five minutes after transplantation, heart grafts received a second injection of 1 × 108 mitochondria in 0.5 mL respiration buffer (Mitochondria) or 0.5 mL respiration buffer (Vehicle) delivered antegrade to the coronary arteries via injection to the coronary ostium using a tuberculin syringe with a 40 G needle. Mice were allowed to recover for 24 hours, and tissue was then collected for further analysis. Serial beating score assessment and echocardiography were obtained for analysis. (B) C57BL/6J male mice (n = 8, 7–9 weeks) were used for sham control (Sham, n = 4). Donor mice heart grafts were immediately transplanted with no CIT and no mitochondrial or respiration buffer injection. Recovery and analysis were identical as mentioned above.