Few insects exhibit the striking color pattern radiation found in bumble bees (Bombus) (1, 2) and some wasps (3, 4). Bumble bees have diversified globally into an unusually wide range of color patterns, many of which converge into high-fidelity Müllerian mimetic complexes across a wide geographic range. Despite extensive documentation of color pattern diversification (1, 2, 5), including a quantitative analysis defining the 12 principal color pattern elements that comprise the phenotype array for all species (2) (Fig. 1A), there has been little understanding of the developmental genetic regulation of pattern formation in bumble bees, hindering progress toward a more general model of the evolution of color pattern mimicry. In PNAS, Tian et al. (6) describe the mechanism by which a bumble bee species can evolve intraspecific dimorphic red/black color patterns. They show that a cis-regulatory region between the Hox genes abdominal-A (abd-A) and Abdominal-B (Abd-B) is responsible for the intraspecific divergence in the black form of the Pacific coastal bumble bee species Bombus melanopygus and the red form found in the intermountain west (Fig. 2).
Fig. 1.
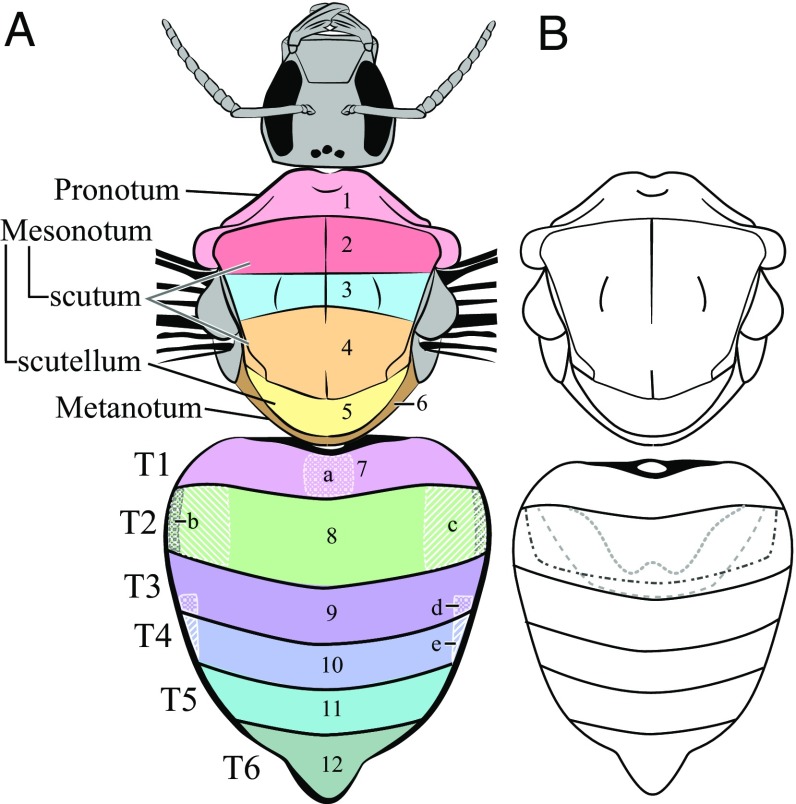
(A) Diagrammatic representation of bumble bee pattern elements 1 to 12 and secondary elements a to e. Each element is labeled; T (tergite) refers to abdominal segments in the bumble bee. (B) Dotted lines on abdominal segment T2 represent some commonly observed species-specific arch-shaped patterns formed of contrasting setae relative to the coloration of the lateral setae.
Fig. 2.
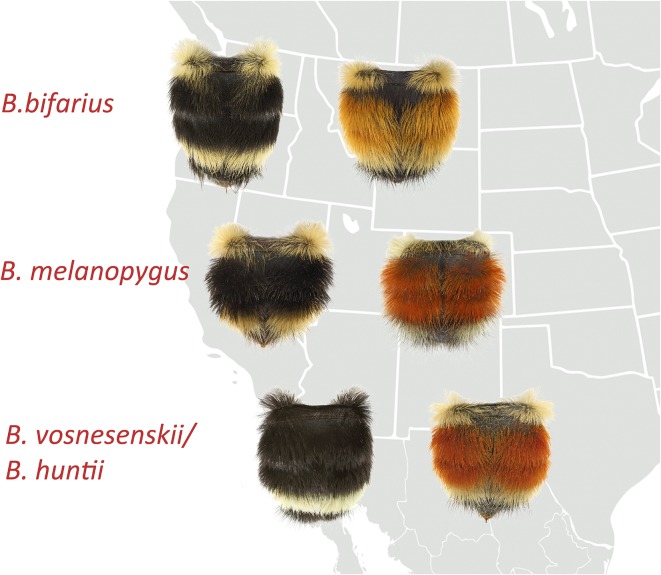
Geographic ranges of sample bumble bee species belonging to the black mimetic complex along the Pacific coast and the red mimetic complex of the intermountain west. This sample group of species reveals both convergent interspecific and divergent intraspecific patterns in which black/red shifts occur in the second and third abdominal segments (e.g., Bombus bifarius and B. melanopygus black forms and red forms). Sister species (e.g., Bombus vosnesenskii and Bombus huntii) may also show the same black/red phenotypic divergence between regions. Photos by Alex Wild. Reprinted from ref. 2, by permission of Oxford University Press.
Tian et al. (6) reveal that the Hox gene Abd-B, which is typically expressed in the posterior 3 abdominal segments of insects, is upregulated in anterior segments (T2 and T3) (Fig. 1) to drive the red/black pigment polymorphism in the midregion of the abdomen in B. melanopygus (Fig. 2). Typically, segments T2 and T3 are controlled by the Hox gene abd-A. However, in T2 and T3 of the red form of B. melanopygus males and females, Abd-B is upregulated 10- to 20-fold in the late stage of pupal development relative to abd-A. Moreover, this late expression occurs only in T2 and T3, clearly indicating the fundamental role of Abd-B in mediating the black/red dimorphic shift. Importantly, Tian et al. (6) show that the temporal expression of Abd-B in T2 and T3 is correlated with the timing of melanin (the red/black pigment) deposition in the setae (hairs) of those segments. The importance of these findings is that a highly pleiotropic gene, such as Hox Abd-B, can shift in expression without deleterious effects if it happens late in development, when the morphological phenotype is set and only cuticular patterns are being expressed. Shifts in the location of red pigmentation, which is found ancestrally on the posterior segments in bumble bees, can now be explained by genetic mutation causing Abd-B to turn on expression of red in the middle abdominal segments late in development. This mechanism of Hox gene mutation in bumble bees could allow relatively rapid shifts in color pattern, thus enabling convergence of new founding populations to a regional color-pattern model. More complex patterns of variation that do not involve the entire segment T2 (and other tergites) (Fig. 1B) remain to be investigated.
It is also notable that Tian et al. (6) find that different independent genetic mutations appear to drive the red/black color pattern shifts on T2 and T3 of convergent closely related bumble bee species with overlapping ranges (Fig. 2). This significant finding is in direct contrast to the well-understood mechanisms of mimetic evolution in Heliconius butterflies, in which the color patterns of closely related comimics evolve from a common ancestral mutation (7).
Together, these exciting findings provide developmental genetic insights for understanding adaptive color pattern mimicry across all bumble bee species and broaden the framework for understanding common genetic mechanisms of color mimicry in other groups of Hymenoptera.
Besides bumble bees, a wide variety of Hymenoptera species, along with some co-occurring insects from other orders, are known to display regional aposematic color-pattern mimicry complexes (see ref. 3; reviewed in ref. 4). Many of these, including hyperdiverse parasitoid wasps, are quite complex in taxonomic composition (8, 9) so that the factors driving the mimicry, and the underlying genetics, are both poorly identified. The elegance of the bumble bee system Tian et al. (6) describe lies in the many replicates among members of the genus Bombus, all of which are likely to share many aspects of their developmental genomic architecture. Independent origins of the color differences can thus be more precisely teased apart at the functional molecular level.
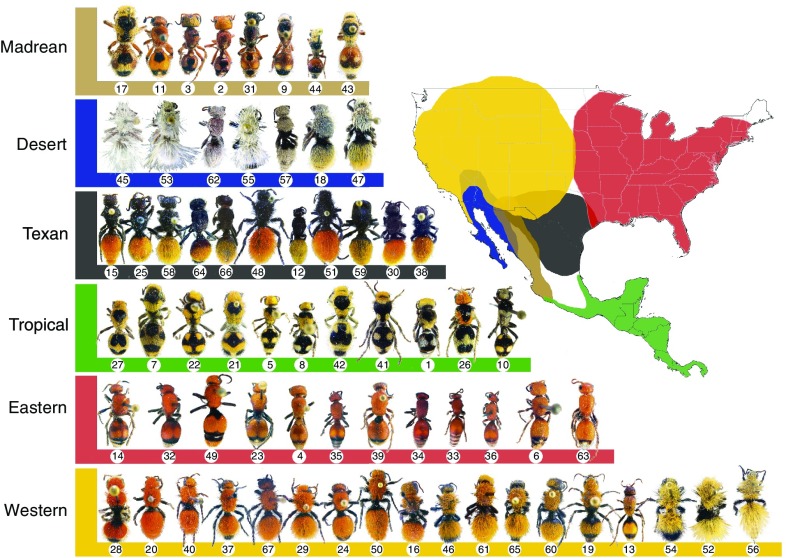
Perhaps the most apt parallel to Bombus within the Hymenoptera is the velvet ant genus Dasymutilla (Mutillidae) (10, 11), where, as in the bumble bees, the color patterns are produced by largely segmental differences in setal pigmentation rather than underlying cuticle color (Fig. 3). Despite the considerable phylogenetic distance between bumble bees and these velvet ants (12), it is likely that they share at least some elements of the developmental mechanism underlying their color patterns. The system described for Bombus by Tian et al. (6) may thus serve as a model system for unlocking the details of Hymenoptera mimicry evolution as a whole.
Fig. 3.
Mimicry rings in velvet ants. Sixty-five North and Central American Dasymutilla velvet ant species (including 3 populations within Dasymutilla bioculata) organized into 6 mimicry rings by shared color, pattern, and geographic region (coded by color). Phylogenetic results (not shown) indicate several independent origins of the color patterns. Reproduced by permission from ref. 11, Springer Nature: Nature Communications, copyright (2012).
Acknowledgments
We thank Sally Corbet for useful comments on the manuscript. S.A.C.’s research is supported by a grant from the US Department of Agriculture, National Institute of Food and Agriculture (2017-67013-26536).
Footnotes
The authors declare no conflict of interest.
See companion article on page 11857.
References
- 1.Williams P. H., The distribution of bumblebee colour patterns world-wide: Possible significance for thermoregulation, crypsis, and warning mimicry. Biol. J. Linn. Soc. Lond. 92, 97–118 (2007). [Google Scholar]
- 2.Rapti Z., Duennes M. A., Cameron S. A., Defining the colour pattern phenotype in bumble bees (Bombus): A new model for evo devo. Biol. J. Linn. Soc. Lond. 113, 384–404 (2014). [Google Scholar]
- 3.Boppré M., Vane-Wright R. I., Wickler W., A hypothesis to explain accuracy of wasp resemblances. Ecol. Evol. 7, 73–81 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quicke D. L. J., Mimicry, Crypsis, Masquerade and Other Adaptive Resemblances (Wiley, Hoboken, NJ, 2017). [Google Scholar]
- 5.Hines H. M., Williams P. H., Mimetic colour pattern evolution in the highly polymorphic Bombus trifasciatus (Hymenoptera: Apidae) species complex and its comimics. Zool. J. Linn. Soc. 166, 805–826 (2012). [Google Scholar]
- 6.Tian L., et al. , A homeotic shift late in development drives mimetic color variation in a bumble bee. Proc. Natl. Acad. Sci. U.S.A. 116, 11857–11865 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hines H. M., et al. , Wing patterning gene redefines the mimetic history of Heliconius butterflies. Proc. Natl. Acad. Sci. U.S.A. 108, 19666–19671 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quicke D. L. J., Preliminary notes on homeochromatic associations within and between the Afrotropical Braconinae (Hym., Braconidae) and Lamiinae (Col., Cerambycidae). Entomol. Mon. Mag. 122, 97–109 (1986). [Google Scholar]
- 9.Quicke D. L. J., et al. , Batesian and Müllerian mimicry between species with connected life histories, with a new example involving braconid wasp parasites of Phoracantha beetles. J. Nat. Hist. 26, 1013–1034 (1992). [Google Scholar]
- 10.Rodriguez J., Pitts J. P., von Dohlen C. D., Wilson J. S., Müllerian mimicry as a result of codivergence between velvet ants and spider wasps. PLoS One 9, e112942 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson J. S., Williams K. A., Forister M. L., von Dohlen C. D., Pitts J. P., Repeated evolution in overlapping mimicry rings among North American velvet ants. Nat. Commun. 3, 1272 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Peters R. S., et al. , Evolutionary history of the Hymenoptera. Curr. Biol. 27, 1013–1018 (2017). [DOI] [PubMed] [Google Scholar]