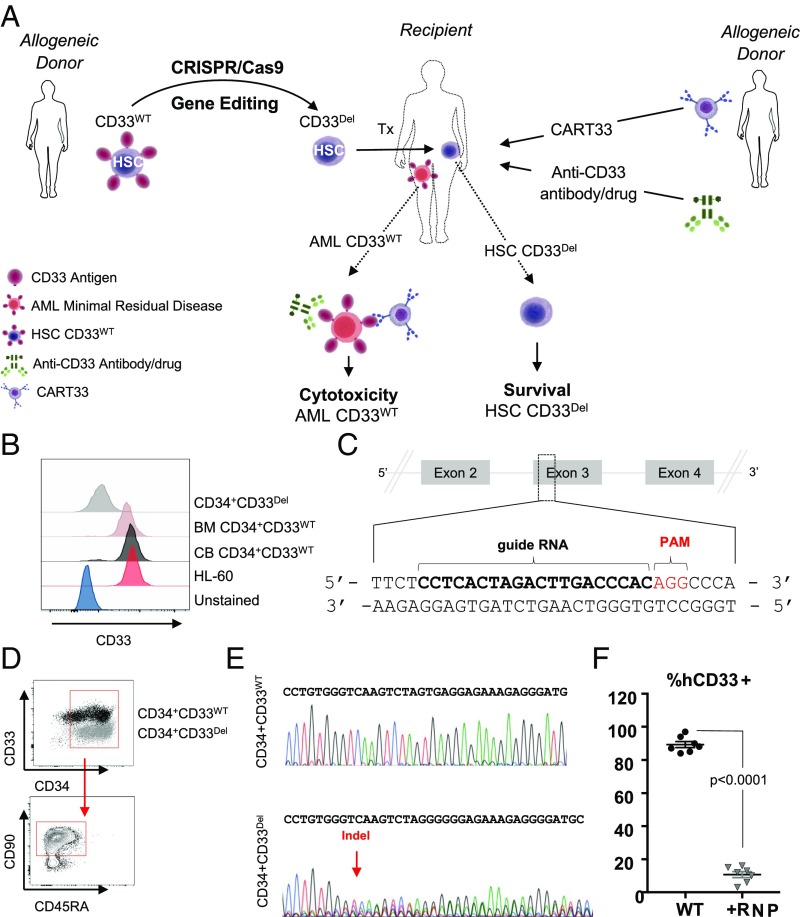
Fig. 1.
(A) Approach: stem cells, either mobilized or CB obtained from a donor, will be genetically manipulated to ablate CD33 expression using gene-editing technology, such as CRISPR/Cas9, and transplanted to relapsed patients eligible for HSCT. Subsequent to transplantation, T cells from an allogeneic donor will be genetically manipulated, using a viral delivery system, to express chimeric antigen receptors targeting CD33 and infused in the recipient. Alternatively, patients can receive ADC (GO) either alone or in combination with CAR-T. (B–F) CD33 expression and its ablation in human cells. (B) Expression of CD33 in human AML cell line HL-60, in human primary CD34+CD33WT cells from BM and CB and human primary CD34+CD33Del after CRISPR/Cas9-mediated ablation. (C) Schematic representation of CD33 genomic locus showing exons 2–4 and location and sequence of sgRNA (in bold, PAM in red) targeting CD33. (D) Surface expression of CD33 by flow cytometry after electroporation in CD34+CD33WT cells and CD34+CD33Del. All cells maintain their stem cells phenotype as assessed by CD90 expression. (E) Chromatogram of Sanger sequencing showing a region surrounding the DNA double-strand break site, (Upper) CD34+CD33WT cells and (Lower) CD34+CD33Del. (F) Five to 7 d after electroporation, CD34+ cultured cells show consistent deletion of CD33 compared with control (15 independent donors).