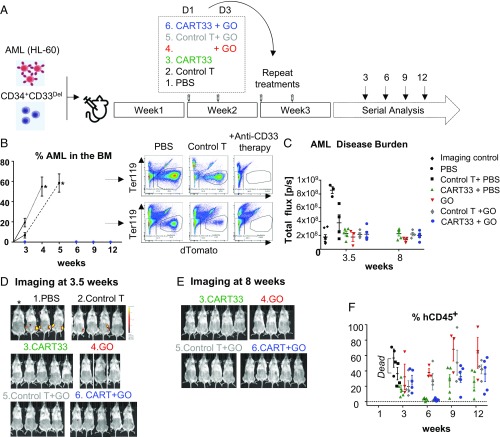
Fig. 5.
Therapy model: CD34+CD33Del cells resist CD33-targeted immunotherapy. (A) Schematic of experimental design: 5 × 105 HL-60 and 5 × 105 CD34+CD33Del were injected in NSGS mice on day 0. One week after, mice were treated with PBS or allogeneic CART33 or control T cells. Three days after a new group received GO only, while allogeneic CART33 and control T cells-injected mice received GO or PBS. Treatment was repeated on week 3. Leukemia progression and CD34+CD33Del engraftment were then monitored by serial BM aspiration. (B) Monitoring of leukemia burden in BM aspirates. Leukemia burden in the whole BM of control groups mice at the time of death are shown with an asterisk (*). Leukemia cells were gated on Ter119−dtomato+. (C) Leukemia burden measure via epifluorescence quantification of images shown in D and SI Appendix, Fig. S5C at 3.5 wk, and E and SI Appendix, Fig. S5D at 8 wk. One mouse representative of each treatment is shown in C and E. See SI Appendix, Fig. S5 for full imaging panel. Background was removed with untreated mouse (*Imaging control). (F) CART33 or GO leukemia clearance doesn’t impair engraftment of CD34+CD33Del cells overtime (% hCD45+ cells), as shown by flow cytometry of BM aspirates. CD34+-injected derived human cells were gated on Ter119−dtomato−, Ly5−/H2kd−human CD45+CART− (three independent experiments with GO, two independent experiments with CART33). Mouse and syringe images designed by Freepik and Kiranshastry from Flaticon.