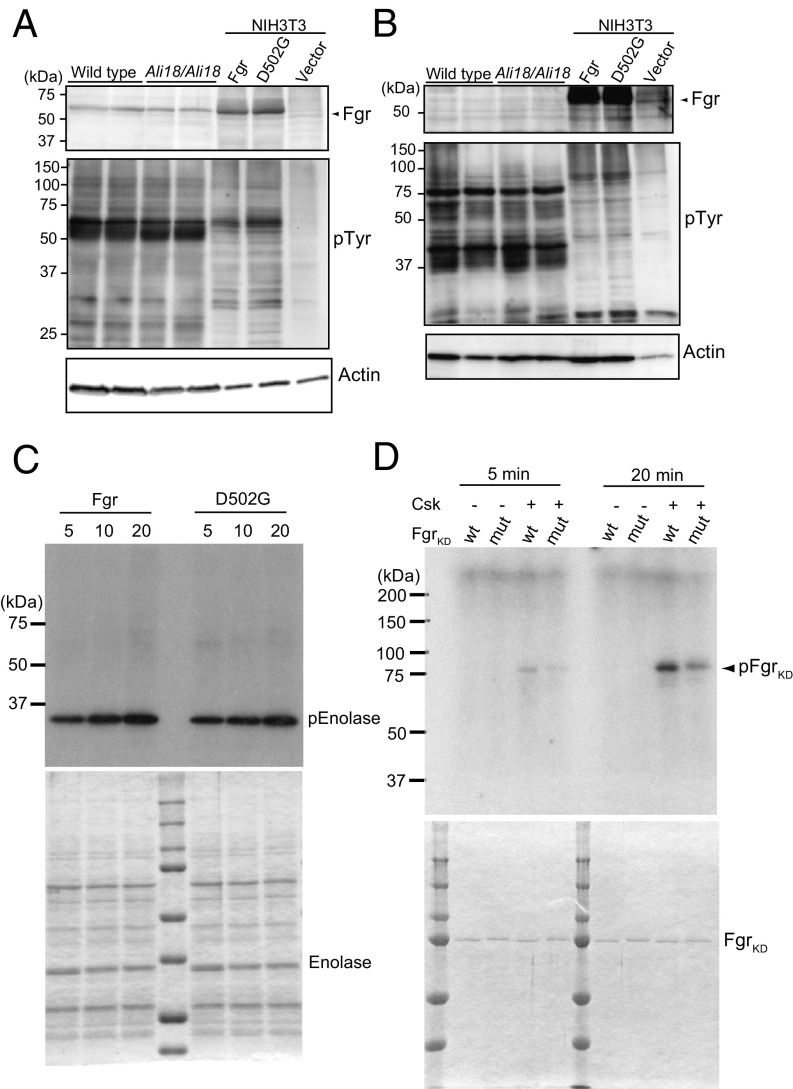
Fig. 3.
Western blot analysis, tyrosine kinase assays, and phosphorylation of Fgr by C-terminal Src kinase (Csk). Cell lysate of spleen (A) and bone marrow cells (B) from wild-type and Ali18/Ali18 mice were used for Western blotting. As control experiments, transformants of wild-type and p.Asp502Gly (D502G) Fgr expression constructs in murine embryo-derived cultured fibroblast, NIH 3T3, cells were used. Empty vector was transfected as negative control. Anti-Fgr (Upper), anti-phosphotyrosine (Middle), and anti-actin (Lower, loading control) were used. No overt changes in Fgr protein levels of Ali18/Ali18 spleen (relative ratio: 0.900 ± 0.256, P = 0.538, t test) and NIH 3T3 cells (relative ratio: 0.982 ± 0.139, P = 0.833, t test) were detected. (C) In vitro translated wild-type and p.Asp502Gly Fgr proteins were used for kinase assay experiments. Recombinant enolase protein was used as SFK-specific substrate (Lower, loading control). No activity changes were detected in different reaction time. (D) The C-terminal phosphorylation of KD Fgr by Csk was measured. Fgr KD with p.Asp502Gly showed five and two times less phosphorylation levels in 5- and 20-min reaction time, respectively. The proteins used for kinase assays were fractionated by SDS/PAGE and stained by Coomassie Brilliant Blue. Experiments were independently triplicated.