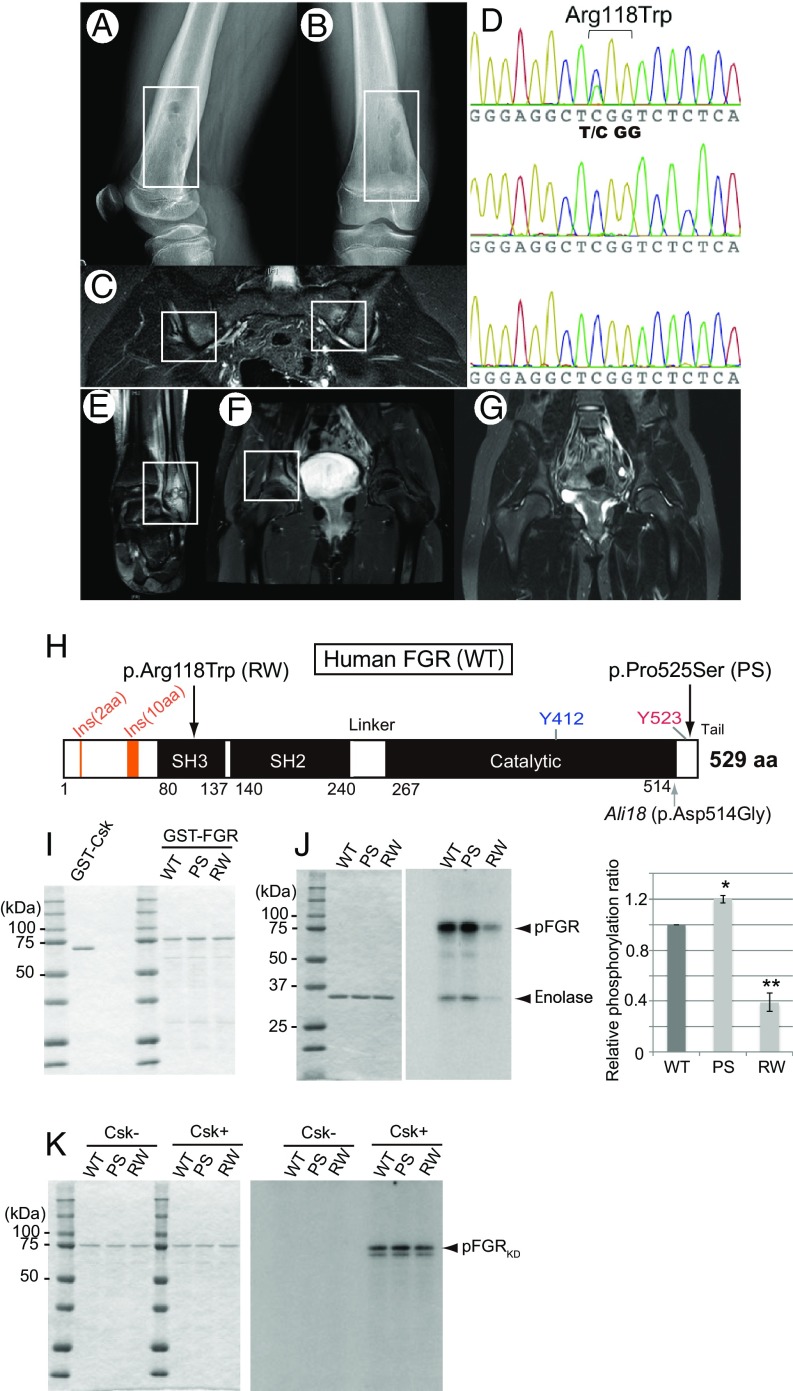
Fig. 4.
Missense mutations of FGR in CRMO and phosphorylation assays. (A and B) Radiograph of case 1 (p.Arg118Trp). Osteolytic lesions with sclerosis and periosteal elevation of the right distal femur are shown. (C) MRI of case 1. Increased STIR signal intensity on the iliac and sacral sides of the left sacroiliac joints is shown. (D) Sanger sequencing chromatogram of p.Arg118Trp in the proband (Top), father (Middle), and mother (Bottom). Proband harbors a de novo C > T mutation, which induces a p.Arg118Trp amino acid change in FGR. (E–G) MRI of case 2 (p.Pro525Ser). Abnormalities included increased signal intensity on STIR images in the left distal fibula (E) and the pelvis at the right acetabulum (F). (G) Repeat MRI 9 mo after naproxen therapy showing improvement in the left acetabular lesion. (H) Schematic diagram of amino acid substitution from the FGR mutations found in human CRMO (Top arrows) and the mouse Ali18 mutation (Lower arrow). (I) SDS/PAGE of affinity purified Csk and FGR used in kinase assays. (J) Kinase activity of FGR with CRMO variants. (J, Left) SDS/PAGE of affinity purified substrate, enolase, used in each assay. (J, Right) Phosphorylation intensity of enolase and FGR indicate enzyme activity. (K) Phosphorylation of FGRKD with CRMO variants by Csk. (Left) SDS/PAGE of affinity purified FGRKD of human CRMO variants as substrate used in each assay. (Right) FGRKD proteins were phosphorylated by Csk in equal intensity. Experiments were independently triplicated.