Figure 7. Proposed model for GSEC‐mediated cleavage of APP and its modulation by GSMs.
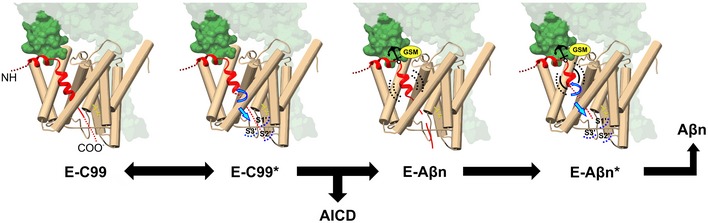
Once the E‐S complex is formed, the first endopeptidase cut strongly destabilizes the helical transmembrane domain of APPC99, leading to the unwinding of the most C‐terminal helical part of the Aβ substrate and providing the length to the “de novo” generated Aβ substrate to reach the active site (model originally proposed in Szaruga et al (2017)). The further unwinding of the Aβ substrate with each sequential cleavage stretches the substrate and provides the length to fill the catalytic pockets but weakens the GSEC‐Aβn interaction, until the eventual E‐S dissociation triggers Aβ release. Here, we propose that the extracellular interface that includes NCT (241/242), APP (K28) and the first extracellular loop of PSEN1 anchors GSEC‐APP/Aβn complexes during the sequential proteolytic mechanism, and the E‐S interface is the target of selected imidazole‐based GSMs.
