Figure 4.
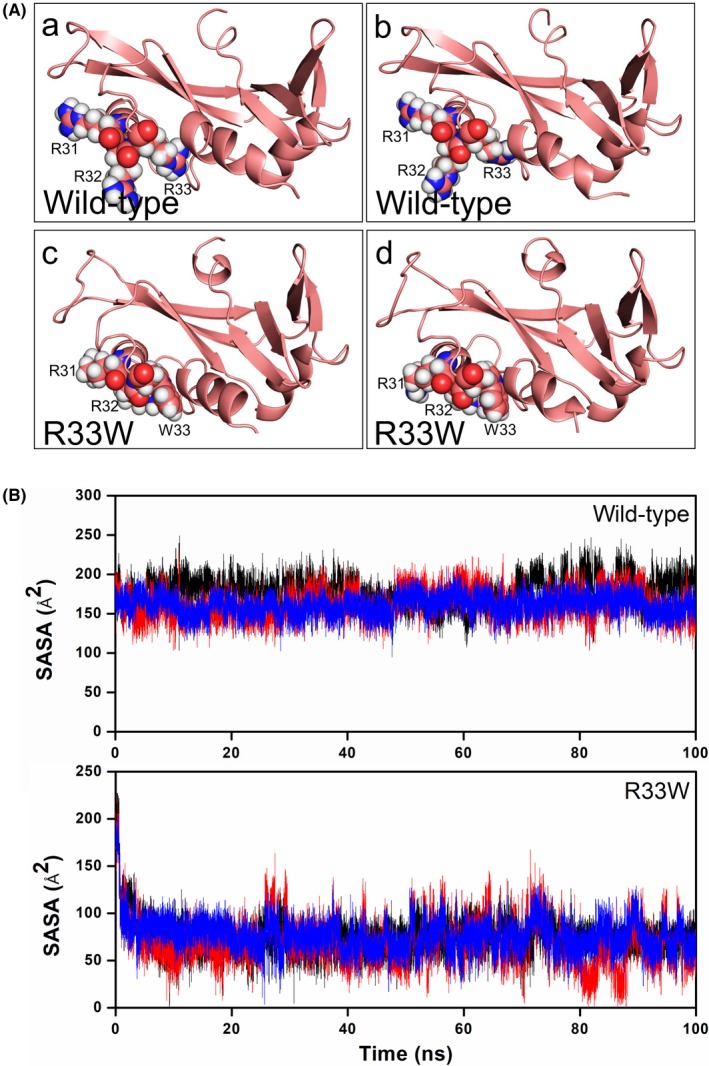
Local folding of nuclear localization signal residues in R33W mutant. (A) Snapshots extracted from molecular dynamics (MD) simulation of wild‐type angiogenin (ANG) (a and b) showing its open conformation and loose packing of 31RRR33 nuclear localization signal residues as compared to the closed and tight packing of 31RRW33 residues (c and d) observed during MD simulations. (B) Computed solvent‐accessible surface area (SASA) from MD simulations of nuclear localization signal residues 31RRR33 or 31RRW33 for wild‐type ANG and R33W mutant, respectively. Reduced SASA in R33W mutant indicates that it may lose its nuclear translocation ability as compared to wild‐type. SASA of R31, R32, and R33/W33 are shown in black, red, and blue, respectively
