Abstract
The kidney collecting duct (CD) is a tubular segment of the kidney where the osmolality and final flow rate of urine are established, enabling urine concentration and body water homeostasis. Water reabsorption in the CD depends on the action of arginine vasopressin (AVP) and a transepithelial osmotic gradient between the luminal fluid and surrounding interstitium. AVP induces transcellular water reabsorption across CD principal cells through associated signaling pathways after binding to arginine vasopressin receptor 2 (AVPR2). This signaling cascade regulates the water channel protein aquaporin-2 (AQP2). AQP2 is exclusively localized in kidney connecting tubules and CDs. Specifically, AVP stimulates the intracellular translocation of AQP2-containing vesicles to the apical plasma membrane, increasing the osmotic water permeability of CD cells. Moreover, AVP induces transcription of the Aqp2 gene, increasing AQP2 protein abundance. This review provides new insights into the transcriptional regulation of the Aqp2 gene in the kidney CD with an overview of AVP and AQP2. It summarizes current therapeutic approaches for X-linked nephrogenic diabetes insipidus caused by AVPR2 gene mutations.
Keywords: Arginine vasopressin, Aquaporin-2, Gene expression regulation, G-protein coupled receptors, Nephrogenic diabetes insipidus
Overview of arginine vasopressin and aquaporin-2
The kidney regulates the balance of water, electrolytes, and acids and bases (pH) in the body. Two critical components for urine concentration in the kidney are: 1) an interstitial osmolality, which provides a driving force for tubular water reabsorption; and 2) the osmotic water permeability of the tubular epithelia, which depends on expression of aquaporin (AQP) water-channel proteins in the cell membrane [1,2]. Consistent with these components, conditions that result in defective urine concentration, such as lithium treatment, are associated with decreased medullary organic osmolytes (e.g., betaine, myoinositol, taurine, and glycerophosphocholine) [3]. Because of high osmotic permeability of water in the tubular epithelia, proximal tubules and descending thin limbs allow the reabsorption of a majority of the water filtered in the glomerulus, where aquaporin-1 (AQP1) mediates near-isosmotic fluid reabsorption [4–6]. AQP1 is also expressed in the descending thin limb and descending vasa recta and facilitates countercurrent exchange in the renal medulla, preventing the dissipation of the salt and urea gradient [7]. The connecting tubule and collecting duct (CD) reabsorb the remaining tubular fluid, which is tightly regulated by arginine vasopressin (AVP) [1,8–11].
Numerous studies have demonstrated that dysregulation of AQPs, solute (co)transporters, and acid-base transporters expressed in renal tubular epithelial cells leads to severe disturbances in water, electrolyte, and acid-base balance and blood pressure [1,10,12,13]. In particular, dysregulation of AVP-mediated water re-absorption in the CD is a primary pathophysiological mechanism underlying disease conditions involving body water disturbances, e.g., systemic water-retaining or water-losing states [1,10,14]. AVP is a peptide hormone produced in the hypothalamus. It is stored in and released from the neurohypophysis. Three exons in the AVP gene encode a signal peptide, AVP, neurophysin II, and copeptin [15]. The Verney receptor in the hypothalamus senses osmotic stimuli and releases AVP in response to a plasma osmolality higher than the physiological threshold (290–295 mOsm/kg H2O) [16,17]. Copeptin, corresponding to the carboxyl terminal portion of provasopressin, is secreted in equimolar amounts to AVP and functions as a stable surrogate marker for AVP secretion [18]. The importance of copeptin measurement was demonstrated in a meta-analytic study of patients with heart failure, which revealed a positive correlation between plasma levels of copeptin and all-cause of mortality [19]. AVP is also secreted in response to non-osmotic stimuli through different pathways such as the parasympathetic afferent pathways [20]. Several factors activate the non-osmotic pathway to secrete AVP, including hypoxia, nicotine, altered hemodynamic states, adrenergic stimuli, adrenal insufficiency, and advanced hypothyroidism [20]. Once secreted into circulation and delivered to the kidney, AVP binds to arginine vasopressin receptor 2 (AVPR2) and induces free water reabsorption in the connecting tubule and CD [1].
In 1992, Lolait et al [21] cloned AVPR2 and subsequently, several studies identified that mutations in the AVPR2 gene on the X-chromosome are associated with X-linked nephrogenic diabetes insipidus (NDI) in humans [21–24]. The exact incidence of X-linked NDI is unknown, however a study showed ~8.8 per million male live births in Quebec, Canada [25]. Autoradiographic localization of 3H-AVP binding is restricted to the medulla of rat kidneys [26] and a study using in situ hybridization demonstrated the distribution of AVPR2 messenger RNA (mRNA) in the outer and inner medulla of rat kidneys [27]. A transcriptome study of microdissected renal tubular segments of rat kidneys confirmed AVPR2 mRNA in the connecting tubule and CD (from the cortical CD to the inner medullary CD); it was also expressed in the thick ascending limb and distal convoluted tubule [28]. Furthermore, V1a receptor (AVPR1a) mRNA is present in the distal convoluted tubule, connecting tubule and cortical CD of rat kidneys [28]. AVPR1a is primarily expressed in the medullary vasculature of the kidneys [29].
In CD principal cells, water reabsorption depends on AVP stimulation. AVP binds to the heterotrimeric G-protein α-subunit (Gαs)-coupled AVPR2 in the basolateral plasma membrane of principal cells and activates adenylyl cyclase 6. This activation increases intracellular cyclic adenosine monophosphate (cAMP) levels, activating kinases and enhancing transcellular water reabsorption in the CD [1,8,9,30–33]. AVPR2 activation by AVP also induces receptor internalization, which is associated with AVPR2 phosphorylation and recruitment of β-arrestin [34]. Aquaporin-2 (AQP2) is a water-channel protein localized in connecting tubule cells and CD principal cells that mediates AVP-induced osmotic water permeability [35,36]. The importance of AVP-regulated AQP2 for urine concentration and body water homeostasis is highlighted in Aqp2 gene null mice and in other clinical conditions in which upregulation or downregulation of AQP2 expression in kidneys is closely associated with water-balance disorders [1,9,10,14,37].
Our studies showed [1,8,9] that AQP2 is regulated on a short-term or long-term basis for water reabsorption in the CD. AQP2 is immunolocalized at the apical plasma membrane and intracellular vesicles of CD principal cells [35]. Short-term regulation is rapidly mediated by AQP2 trafficking from intracellular vesicles to the apical plasma membrane [1,35,38,39]. The intracellular translocation of AQP2 to the apical plasma membrane is associated with phosphorylation of a serine residue in the carboxyl terminus of AQP2 via activation of the cAMP/ protein kinase A (PKA) signaling pathway [39–41]. Long-term regulation or adaptation of CD water permeability following AVP stimulation is mediated by changing the half-life and abundance of AQP2 protein [42–45]. The abundance of AQP2 protein is regulated by transcription of the Aqp2 gene and translation, and post-translational modification of products, including ubiquitination and subsequent proteasomal and/or lysosomal degradation, which involve the actions of E3 ubiquitin-protein ligases (e.g., NEDD4 and CHIP) [43,46–52]. In addition, microR-NAs (miRNA; e.g., miR-32 and miR-137) are important post-transcriptional modulators that regulate AQP2 protein abundance [53,54]. A recent study showed that miR-132 regulates hypothalamic AVP mRNA expression [55]. Methyl-CpG-binding protein-2 is a target of miR-132 that inhibits hypothalamic AVP synthesis by binding the methyl-CpG-binding protein-2 enhancer region [55].
NDI represents the inability of kidneys to concentrate urine despite AVP stimulation [1,10,11,56–58]. NDI is caused by genetic defects, with primary inherited forms caused by mutations in the AVPR2 or AQP2 genes, or acquired conditions. Secondary acquired forms are caused by drugs, electrolyte disturbances, renal failure, and other diseases [10,25,56,59–62]. In humans, AVP can concentrate urine and reduce the urine flow rate to ~0.7 L/day and increase urine osmolality to ~1,200 mOsm/kg H2O [63]. In contrast, in the absence of AVP and AQP2 response, the urine flow rate can increase up to ~28 L/day and urine osmolality can decline to 50 mOsm/kg H2O [62]. Thus, NDI is associated with AVP-resistant severe polyuria, dehydration, and electrolyte disturbance. This review presents new insights on the transcriptional regulation of the Aqp2 gene and summarizes novel approaches for the treatment of hereditary NDI, particularly when caused by genetic defects in the AVPR2 gene.
Regulation of Aqp2 gene transcription
The regulatory mechanisms involved in the AVP-mediated increase of AQP2 protein, as a long-term response to AVP stimulation for hours or even days, have been widely studied. Two independent studies showed that AVP increases AQP2 mRNA and protein in inner medullary collecting duct (IMCD) cells isolated from rat kidneys [64,65]. In contrast, water loading decreases AQP2 mRNA and protein in rats continuously treated with dDAVP (an AVPR2-selective agonist), demonstrating “vasopressin escape” [42,66]. These studies on AVP-dependent or AVP-independent regulation of AQP2 abundance led to other studies on Aqp2 transcription. AVP increases intracel-lular cAMP levels and activity of cAMP-responsive PKA by activating G protein-coupled receptor (GPCR) AVPR2, resulting in increased Aqp2 expression and AQP2 insertion into the apical plasma membrane [8,33,38,39,67,68]. Hozawa et al [69] and Yasui et al [31] demonstrated that cAMP-responsive elements (CREs) within 350 bp upstream of the transcription start site of Aqp2 in rat kidney IMCD cells are critical regulatory elements in AVP-mediated Aqp2 transcription. Matsumura et al [30] confirmed impaired activity of the Aqp2 promoter following deletion of the CREs. Consistent with those results was a study showing that knocking out both catalytic PKA subunits α and β (encoded from Prkaca and Prkacb) abolished expression of AQP2 mRNA and protein in mpkCCD cells treated with dDAVP [41]. These results demonstrate that cAMP/PKA signaling is a critical regulatory pathway for AVP-mediated AQP2 expression in the renal CD. However, other stimuli that regulate AQP2 expression have also been found under certain physiological and pathophysiological conditions (Table 1) [53,66,70–77]. For example, Kortenoeven et al [78] demonstrated that activation of cAMP-Epac, a guanine exchange factor directly activated by cAMP [79], is more important than the PKA-CRE pathway in the long-term regulation of AQP2. These findings suggest the need for more studies to further understand the transcription regulators of Aqp2.
Table 1.
Regulatory mechanisms of AQP2 trafficking/expression in the renal collecting duct
| Regulator | Regulation | Mechanism | Components |
|---|---|---|---|
| Hormones | Trafficking/ Expression | Signaling pathway activation | Vasopressin, oxytocin, angiotensin II, aldosterone, secretin, calcitonin, and their receptors |
| Kinases | Trafficking/ Expression | Signal transduction | cAMP/PKA, PI3K/Akt/AS160, MAPK (ERK, JNK, p38), GSK-3β, CaMKII, AMPK, Epac, and extracellular matrix-to-intracellular scaffold protein ILK |
| Transcription factors | Expression | Transcription | CREB family, c-Jun and c-Fos heterodimer (AP-1) and Rel family members, NF-κB, and NFAT subfamily |
| Cellular signaling | Trafficking/ Expression | Protein-protein interaction | (1) Between AQP tetramers. (2) Between AQP monomers. (3) Transient interactions with regulatory proteins: clathrin heavy chain; Hsc70; annexin II; LIP5; cytoskeletal or cytoskeleton-associated proteins such as actin, tropomyosin 5b, and ezrin; PDZ domain-containing protein, such as SPA-1 and Sipa1I1; and retromer complex (Vps35) |
| Protein-modification enzymes | Trafficking/ Expression | Post-translational modification | Phosphorylation, ubiquitination (E3 ligases), deubiquitination, glycosylation, and glutathionylation |
| Receptors/Agonists | Trafficking/ Expression | Signaling pathway activation | AVPR2, angiotensin II AT1a receptor, prostanoid receptor (EP2, EP4), frizzled receptor, β3-adreneroreceptor, serotonin receptor, calcitonin receptor, calcium-sensing receptor, epidermal growth factor receptor, bile acid receptor-coupled GPCR, and purinergic receptor |
| Extracellular microenvironment | Trafficking | Post-translational modification, cytoskeletal rearrangement | Tubular flow, medullary tonicity, and extracellular pH |
| MicroRNAs | Expression | RNA interference | AQP2-targeting microRNAs (miR-32, miR-137) |
Akt, protein kinase B; AMPK, 5′ adenosine monophosphate-activated protein kinase; AP-1, activator protein 1; AS160, Akt substrate of 160 kDa; AVPR2, arginine vasopressin receptor 2; CaMKII, calcium/calmodulin-dependent protein kinase II; cAMP, cyclic adenosine monophosphate; CREB, cAMP response element-binding protein; EP2, prostaglandin E2 receptor 2; EP4, prostaglandin E2 receptor 4; Epac, guanine exchange factor directly activated by cAMP; ERK, extracellular signal-regulated kinase; GPCR, G protein-coupled receptor; GSK-3β, glycogen synthase kinase-3β; Hsc70, heat shock cognate protein 70; ILK, integrin-linked kinase; JNK, c-Jun N-terminal kinase; LIP5, lysosomal trafficking regulator-interacting protein 5; MAPK, mitogen-activated protein kinase; PI3K, phosphoinositide 3-kinase; PKA, protein kinase A; Sipa1I1, signal-induced proliferation-associated 1 like 1; SPA-1, signal-induced proliferation-associated gene-1; Vps35, vacuolar protein sorting-associated protein 35.
The binding of transcription factors (TFs) and cofactors to enhancers can stimulate the transcription of an associated gene [80]. A recent study using high-throughput next-generation sequencing (NGS) techniques (ChIP-seq and ATAC-seq) revealed potential enhancer elements for Aqp2 in the mouse cortical CD cell line mpkCCD [81–83]. Two enhancer elements were identified within a topologically associating domain containing Faim2-Aqp2-Aqp5 genes, which is a CTCF-insulated loop regulated by a TF CTCF homodimer [84]. Although further functional studies are required to fully understand the roles of the identified enhancer elements in Aqp2 transcription, this study provides insights into Aqp2 transcription regulation mediated by genomic regulatory elements.
Promoters and enhancers are genomic regulatory elements with multiple TF-binding sites that facilitate transcription initiation mediated by transcriptional regulators [85]. Transcriptional regulation via a combination of TFs is complicated. These cell type-specific processes exist due to differential expression levels of TFs in various cell types. Thus, investigation of TF-binding sites and regulatory proteins can provide direct information about mechanisms that regulate Aqp2 transcription in the CD. In-silico analyses using bioinformatic tools indicate several potential conserved TF motifs found by scanning for TF consensus motif sequences along the genomic DNA of the Aqp2 promoter [86–89]. However, sequence-specific DNA-binding TFs contain a conserved DNA-binding domain across the family with shared consensus DNA motifs in the genome, as shown in bioinformatic analysis by Hwang et al [89].
Many studies using in vitro and in vivo models identified TFs that regulate Aqp2 transcription. Transcriptional activity of the Aqp2 promoter mediated by specific TFs such as ELF3, ELF5, and GATA2 [86,90,91] has been evaluated using luciferase reporter assays. Some TF-knockout mice, such as mice deficient in GATA2 or farnesoid X receptor (FXR), show reduced Aqp2 transcription with impaired urinary concentration [91,92]. Tonicity regulates Aqp2 transcription mediated by TonEBP and Epac, but independent of AVP [93,94]. A study on tonicity-responsive AQP2 expression revealed that a calcium-dependent calcineurin-NFATc pathway also involves increased AQP2 mRNA expression [95]. An in vitro tubulointerstitial inflammation model of mpkCCD cells induced by lipo-polysaccharides (LPS) showed that LPS-activated nuclear factor kappaB (NF-κB) reduces AQP2 mRNA expression [96,97].
High-throughput NGS techniques for genome-wide identification of TF binding sites, namely ChIP-seq (chromatin immunoprecipitation followed by NGS), provides direct evidence for the presence of TF-binding sites that could regulate Aqp2 transcription. A recent ChIP-seq analysis of mpkCCD cells identified a binding site of TF C/EBPβ, which is known as a pioneer TF, 400 bp downstream of Aqp2 [81]. Several high-throughput NGS techniques such as ChIP-seq, ChIP-exo, and cut-and-run, as well as conventional ChIP-PCR methods are important tools for further studies to identify TFs that bind to genes of interest [98].
Genomic regions associated with gene expression are also regulated directly or indirectly by cofactors. Post-translational histone modifications by histone-modifying enzymes can alter chromatin structure [99]. Histone modifications at the N-terminal tails of histones, including methylation, phosphorylation, acetylation, ubiquitylation, and SUMOylation, lead to dynamic changes in chromatin structure and gene transcription [100,101]. Therefore, histone modifications in the vicinity of a gene mark its transcription status. For instance, a ChIP-seq analysis in mpkCCD cells showed that histone H3 acetylation at Lys27 (H3K27Ac) markedly increases at the Aqp2 promoter following dDAVP treatment [41]. Moreover, an increase in H3K27 acetylation levels at the Aqp2 promoter, which indicates activation, is consistent with enhanced binding of RNA polymerase II at the promoter and increased AQP2 mRNA expression [43]. However, precise cofactors such as acetyltransferases/deacetylases that directly modify histones and TFs at the Aqp2 promoter region remain to be elucidated.
Lysine acetyltransferase CREB-binding protein (CBP, gene symbol: Crebbp) and P300 (gene symbol: Ep300) are transcription coactivating factors that acetylate histones and TFs [102,103]. A study showed that CBP and P300 cooperate with β-catenin [104]. CBP acetylates β-catenin at Lys49, leading to promoter-specific gene expression. Interestingly, β-catenin has been studied as a potential AVP-responsive transcription regulator. Protein mass spectrometry analysis of renal CDs showed that AVP increased phosphorylation of β-catenin at Ser552 and its translocation into the nucleus in CD cells [87,105–107]. Moreover, siRNA-mediated knockdown of β-catenin significantly impairs dDAVP-induced AQP2 expression in mpkCCD cells [107].
Beyond transcription regulatory proteins associated with genomic regulatory elements, chromatin modifications such as DNA methylation are potential epigenetic regulatory mechanisms of Aqp2 expression. Several studies using bisulfite sequencing of targeted genomic regions or whole genomes identified methylated cytosines within CpG islands, which are called the DNA methylome. These studies reported that DNA methylation widely regulates gene expression in kidney cells [108,109] or tissues obtained from models of renal ischemia-re-perfusion injury [110] and hypertension [111]. Aqp2 has a CpG island in the fourth exon. However, the regulation of Aqp2 expression associated with DNA methylation has not been explored yet.
Current approaches for treating AVPR2 mutation-induced X-linked NDI
Hereditary NDI is a genetic disease caused by mutations in the AVPR2 or AQP2 genes [22,25,56,59–62]. Gene mutations in AVPR2 result in X-linked NDI, which is the most common (~90%) form of inherited NDI [22,56,61,112,113]. To date, more than 250 different AVPR2 mutations have been identified in more than 300 families (The Human Gene Mutation Database at the Institute of Medical Genetics in Cardiff; http://www.hgmd.cf.ac.uk) [56,114]. Deen and his associates [115] classified AVPR2 gene mutations into five categories: 1) Class I indicates absence of AVPR2 protein due to defects in transcription, mRNA processing, or translation (e.g., promoter alterations, aberrant splicing, exon skipping, and most frameshift and nonsense mutations); 2) Class II indicates retention of fully translated AVPR2 proteins in the endoplasmic reticulum (ER) due to misfolding of AVPR2, preventing localization at the plasma membrane. The underlying mutations include missense, insertion, or deletions. Class II mutations are the most common form of AVPR2 defect [11,56,57]. The treatment for many patients with X-linked NDI requires restoring plasma membrane expression of mutant AVPR2 [116,117]; 3) Class III indicates misfolding of AVPR2 leading to defective functions (e.g., impaired G protein binding and intracellular signaling), despite correct transport to the plasma membrane; 4) Class IV has no apparent defects in protein trafficking to the plasma membrane, but decreased binding affinity to AVP; 5) Class V indicates improper protein sorting to intracellular structures, e.g., β-arrestin-positive intracellular vesicles [118].
To treat NDI caused by AVPR2 gene mutations (Table 2), the first strategy is restoration of AVPR2 plasma membrane expression using chemical chaperones (e.g., glycerol and dimethyl sulfoxide), since AVPR2 mutations are fully functional, but misfolded AVPR2 protein is retained in the ER/Golgi (Class II) [119]. Previous studies demonstrated that several cell-permeable AVPR2 antagonists (pharmacological chaperones such as S121463, VPA-985, SR49059, conivaptan, OPC31260, and OPC41061) stabilize AVPR2 mutants in the ER and allow their escape for plasma membrane expression [116,120–122]. Cell-permeable, nonpeptide AVPR2 agonists (e.g., VA999088, VA999089, and OPC51803) bind intracellularly retained AVPR2 and activate a signaling pathway to induce cAMP accumulation without translocating receptors to the plasma membrane [123,124]. Other nonpeptide agonists (e.g., MCF14, MCF18, and MCF57) activate AVPR2 and translocate the receptors to the plasma membrane [125]. However, AVPR2 mutations resulting in the absence of full-length AVPR2 protein (Class I promoter alterations, aberrant splicing, exon skipping, and most frameshift and nonsense mutations) cannot be treated by these approaches.
Table 2.
Potential therapeutic strategies for X-lined NDI associated with AVPR2 mutation
| Evidence for therapeutic strategies |
|---|
|
AQP2, aquaporin-2; AVP, arginine vasopressin; AVPR2, arginine vasopressin receptor 2; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; EGFR, epidermal growth factor receptor; EP2, prostaglandin E2 receptor 2; EP4, prostaglandin E2 receptor 4; ER, endoplasmic reticulum; GPCR, G protein-coupled receptor; NDI, nephrogenic diabetes insipidus; PDE, phosphodiesterase; PDE5, PDE type 5; RhoA, Ras homolog gene family member A; TGR5, G-protein coupled bile acid receptor 1 (GPBAR1).
Another therapeutic approach relies on AVP-independent AQP2 trafficking to the plasma membrane. This can be achieved through bypassing AVPR2 signaling and inducing AQP2 accumulation in the membrane by translocating AQP2. These approaches have two categories: 1) intracellular cAMP elevation by activating other GPCRs or inhibiting phosphodiesterases (PDEs); and 2) cAMP-independent pathways. For elevating intracel-lular cAMP levels, studying endogenously expressed GPCRs in addition to AVPR2 in the renal CD is important. GPCRs naturally couple to GαS to increase cAMP levels and regulate AQP2 expression. The potential candidate GPCRs include the prostaglandin E receptors (EP2 and EP4), β3-adrenergic receptor (β3-AR), calcitonin receptor, secretin receptor, and TGR5 (a bile acid-activated membrane receptor) [10,77,126–132]. Li et al [129] demonstrated that an EP4 agonist increases cAMP levels in mouse kidney IMCD suspensions, increasing urine osmolality and decreasing urine volume in conditional Avpr2-knockout mice. Moreover, treatment with the EP2 agonist, butaprost, significantly decreases urine volume in rats pretreated with an AVPR2 antagonist OPC [130]. The β3-adrenergic receptor was immunolocalized at the basolateral plasma membrane of tubular epithelial cells in the thin and thick ascending limbs, distal convoluted tubule, and cortical and medullary CD, where AVPR2 is mainly expressed [126]. The same study demonstrated that the β3-AR agonist BRL37344 increases cAMP production in mouse kidney tubule suspensions and decreases urine output in mice that do not express functional AVPR2. Calcitonin induces increases in intracellular cAMP and AQP2 trafficking in AQP2-expressing LLC-PK1 cells. This increase is dependent on cAMP-PKA activity [132]. Calcitonin treatment in AVP-deficient Brattleboro rats is antidiuretic during the first 12 hours of treatment, although this effect is attenuated long-term [132]. Intravenous administration of secretin in rats decreases urine output [128]. Chu et al [127] demonstrated that secretin receptor-null mice have mild polydipsia and polyuria associated with reduced expression of AQP2 and AQP4 in the kidney. Another study further demonstrated that secretin increases intracellular cAMP levels in mouse IMCD tubule suspensions and that chronic infusion of secretin in Avpr2 gene-deficient mice increases AQP2 mRNA and protein [133]. Furthermore, activation of bile acid receptor-coupled GPCR (TGR5) affects AQP2 trafficking and protein expression in inner medullary CD cells via a cAMP-PKA signaling pathway [77]. Importantly, TGR5 stimulation improves impaired urine concentration in mice with lithium-induced NDI by increasing AQP2 protein abundance [77]. These data indicate that in addition to AVPR2 in the renal CD, activation of endogenously expressed GPCRs that naturally couples to GαS increases cAMP levels and regulates AQP2 expression and water re-absorption. In addition, AQP2 trafficking and abundance are affected by the cAMP-Epac pathway [78,134]. Since Epac is activated by a cAMP analog (8-pCPT-2′-O-Me-cAMP), Epac activators could be used to increase AQP2 expression in the membrane.
Inhibition of PDE is another strategy to induce intracellular cAMP levels. Sohara et al [60] showed that the PDE4 inhibitor rolipram increases urine osmolality in mutant AQP2 (763–772 del) knockin mice associated with increased cAMP levels in renal papillae. Another study demonstrated that cotreatment with PDE3 (milrinon) and PDE4 (rolipram) inhibitors reduces impaired urinary concentration ability in rats with hypercalcemia [135]. An additional study showed that increasing cGMP levels via PDE5 inhibitors or cGMP induces AVP-independent AQP2 trafficking to the plasma membrane. Consistent with these results, the PDE5 inhibitor sildenafil citrate induces AQP2 redistribution to the apical plasma membrane in rat kidney slices and reduced polyuria in rats with lithium-induced NDI [136,137].
An alternative approach is to find GPCRs in the kidney CD that do not couple to the GαS and cAMP pathway but regulate AQP2 expression. Ando et al [138] demonstrated that Wnt5a, a ligand for frizzled receptors, activates in-tracellular calcium release. The calcium-binding protein calmodulin and calmodulin-mimicking protein AA stimulate calcineurin, which decreases AQP2 phosphorylation at S261 and increases phosphorylation at S269, leading to AQP2 apical trafficking [138]. Wnt5 administration to mice pretreated with tolvaptan, an AVPR2 antagonist, increases urine osmolality and induces AQP2 trafficking to the apical plasma membrane [138]. Epidermal growth factor receptor (EGFR) inhibitors do not affect intracellular levels of cAMP and cGMP in LLC-AQP2 cells [139]. However, EGFR inhibitors increase AQP2 membrane accumulation in LLC-PK1 cells, reduce urine volume and increase urine osmolality in mice with lithium-induced NDI [139]. These results indicate that frizzled receptor and EGFR could be involved in AQP2 regulation independent of the AVP-cAMP/PKA pathway. An additional study demonstrated that tamoxifen, a selective estrogen receptor modulator, improves impaired urine concentration and reduces downregulation of AQP2 protein abundance in rats with lithium-induced NDI, although the underlying mechanisms are still unclear [140]. However, tamoxifen is unlikely to increase intracellular cAMP levels (unpublished data). Similarly, metformin, an AMPK activator, increases urine osmolality and AQP2 abundance in rats with AVPR2 blockade by tolvaptan treatment and Avpr2 null mice [141], suggesting a complex mode of AQP2 regulation.
Another strategy is inhibition of AQP2 internalization. Treatment with statins can prevent AQP2 internalization and induce AQP2 accumulation at the plasma membrane [142]. Simvastatin is associated with increased apical membrane AQP2 expression in cultured cells and kidney slices from Brattleboro rats [142]. Fluvastatin increases AQP2 expression in the apical plasma membrane of the kidney CD in C57BL/6 mice [143] and a combination treatment of fluvastatin and secretin dramatically decreases urine output in Avpr2 null mice [133]. These results suggest that statin treatment could be used to increase urine concentration in patients with X-linked NDI. Future clinical studies will confirm the efficacy of statin treatment in patients with hereditary NDI.
Summary and perspectives
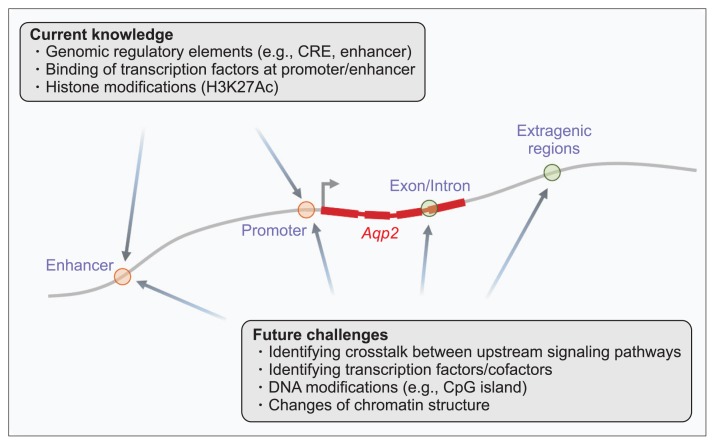
Water reabsorption in the CD is regulated by the action of AVP. AVP stimulates the subcellular trafficking of AQP2-expressing vesicles to the apical plasma membrane, inducing osmotic water permeability in CD principal cells. Moreover, AVP activates Aqp2 transcription, increasing AQP2 protein abundance. Hereditary NDI is a genetic disease caused by mutations in the AVPR2 or AQP2 genes. AVPR2 gene mutations result in X-linked NDI, the most common form of inherited NDI. For treatment, restoration of AVPR2 expression in the plasma membrane using chemical chaperones or activation of intracellularly retained AVPR2 using cell-permeable AVPR2 agonists is suggested. Several approaches for bypassing AVPR2 signaling and inducing membrane AQP2 accumulation, i.e., AVP-independent AQP2 trafficking to the plasma membrane, have been demonstrated. Future approaches to treatment should aim to fully understand the mechanisms of Aqp2 transcription under various physiological and pathophysiological conditions. A multiomics approach could provide comprehensive insights into: 1) transcriptional regulation via transcription regulatory complexes, 2) genomic regulatory elements, and 3) alterations in signaling pathways in the X-linked hereditary NDI (Fig. 1).
Figure 1. Future approaches to understanding the mechanisms of Aqp2 gene transcription.
A multiomics approach could provide comprehensive insights into transcriptional regulation cooperated by transcription regulator complexes, genomic regulatory elements, and signaling pathway crosstalk in X-linked hereditary nephrogenic diabetes insipidus. CRE, cyclic adenosine monophosphate-responsive elements.
Acknowledgments
This research was supported by Kyungpook National University Research Fund, 2018.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Hyun Jun Jung and Tae-Hwan Kwon participated in the conception and wrote the manuscript. All authors read and approved the final manuscript.
References
- 1.Knepper MA, Kwon TH, Nielsen S. Molecular physiology of water balance. N Engl J Med. 2015;372:1349–1358. doi: 10.1056/NEJMra1404726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi HJ, Yoon YJ, Kwon YK, et al. Patterns of gene and metabolite define the effects of extracellular osmolality on kidney collecting duct. J Proteome Res. 2012;11:3816–3828. doi: 10.1021/pr300309d. [DOI] [PubMed] [Google Scholar]
- 3.Hwang GS, Yang JY, Ryu DH, Kwon TH. Metabolic profiling of kidney and urine in rats with lithium-induced nephrogenic diabetes insipidus by (1)H-NMR-based metabonomics. Am J Physiol Renal Physiol. 2010;298:F461–F470. doi: 10.1152/ajprenal.00389.2009. [DOI] [PubMed] [Google Scholar]
- 4.Schnermann J, Chou CL, Ma T, Traynor T, Knepper MA, Verkman AS. Defective proximal tubular fluid reabsorption in transgenic aquaporin-1 null mice. Proc Natl Acad Sci U S A. 1998;95:9660–9664. doi: 10.1073/pnas.95.16.9660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. CHIP28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol. 1993;120:371–383. doi: 10.1083/jcb.120.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallon V, Verkman AS, Schnermann J. Luminal hypotonicity in proximal tubules of aquaporin-1-knockout mice. Am J Physiol Renal Physiol. 2000;278:F1030–F1033. doi: 10.1152/ajprenal.2000.278.6.F1030. [DOI] [PubMed] [Google Scholar]
- 7.Pallone TL, Turner MR, Edwards A, Jamison RL. Counter-current exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1153–R1175. doi: 10.1152/ajpregu.00657.2002. [DOI] [PubMed] [Google Scholar]
- 8.Jung HJ, Kwon TH. Molecular mechanisms regulating aquaporin-2 in kidney collecting duct. Am J Physiol Renal Physiol. 2016;311:F1318–F1328. doi: 10.1152/ajprenal.00485.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwon TH, Frøkiær J, Nielsen S. Regulation of aquaporin-2 in the kidney: a molecular mechanism of body-water homeostasis. Kidney Res Clin Pract. 2013;32:96–102. doi: 10.1016/j.krcp.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen S, Frøkiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev. 2002;82:205–244. doi: 10.1152/physrev.00024.2001. [DOI] [PubMed] [Google Scholar]
- 11.Moeller HB, Rittig S, Fenton RA. Nephrogenic diabetes insipidus: essential insights into the molecular background and potential therapies for treatment. Endocr Rev. 2013;34:278–301. doi: 10.1210/er.2012-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossier BC, Bochud M, Devuyst O. The hypertension pandemic: an evolutionary perspective. Physiology (Bethesda) 2017;32:112–125. doi: 10.1152/physiol.00026.2016. [DOI] [PubMed] [Google Scholar]
- 13.Kwon TH, Laursen UH, Marples D, et al. Altered expression of renal AQPs and Na(+) transporters in rats with lithium-induced NDI. Am J Physiol Renal Physiol. 2000;279:F552–F564. doi: 10.1152/ajprenal.2000.279.3.F552. [DOI] [PubMed] [Google Scholar]
- 14.Kortenoeven ML, Fenton RA. Renal aquaporins and water balance disorders. Biochim Biophys Acta. 2014;1840:1533–1549. doi: 10.1016/j.bbagen.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Burbach JP, Luckman SM, Murphy D, Gainer H. Gene regulation in the magnocellular hypothalamo-neurohypophysial system. Physiol Rev. 2001;81:1197–1267. doi: 10.1152/physrev.2001.81.3.1197. [DOI] [PubMed] [Google Scholar]
- 16.Verney EB. The antidiuretic hormone and the factors which determine its release. Proc R Soc Lond B Biol Sci. 1947;135:25–106. doi: 10.1098/rspb.1947.0037. [DOI] [PubMed] [Google Scholar]
- 17.Zerbe RL, Robertson GL. Osmoregulation of thirst and vasopressin secretion in human subjects: effect of various solutes. Am J Physiol. 1983;244:E607–E614. doi: 10.1152/ajpendo.1983.244.6.E607. [DOI] [PubMed] [Google Scholar]
- 18.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem. 2006;52:112–119. doi: 10.1373/clinchem.2005.060038. [DOI] [PubMed] [Google Scholar]
- 19.Zhong Y, Wang R, Yan L, Lin M, Liu X, You T. Copeptin in heart failure: review and meta-analysis. Clin Chim Acta. 2017;475:36–43. doi: 10.1016/j.cca.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Schrier RW, Berl T, Anderson RJ. Osmotic and nonosmotic control of vasopressin release. Am J Physiol. 1979;236:F321–F332. doi: 10.1152/ajprenal.1979.236.4.F321. [DOI] [PubMed] [Google Scholar]
- 21.Lolait SJ, O’Carroll AM, McBride OW, Konig M, Morel A, Brownstein MJ. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- 22.Rosenthal W, Seibold A, Antaramian A, et al. Molecular identification of the gene responsible for congenital nephrogenic diabetes insipidus. Nature. 1992;359:233–235. doi: 10.1038/359233a0. [DOI] [PubMed] [Google Scholar]
- 23.van den Ouweland AM, Dreesen JC, Verdijk M, et al. Mutations in the vasopressin type 2 receptor gene (AVPR2) associated with nephrogenic diabetes insipidus. Nat Genet. 1992;2:99–102. doi: 10.1038/ng1092-99. [DOI] [PubMed] [Google Scholar]
- 24.Pan Y, Metzenberg A, Das S, Jing B, Gitschier J. Mutations in the V2 vasopressin receptor gene are associated with X-linked nephrogenic diabetes insipidus. Nat Genet. 1992;2:103–106. doi: 10.1038/ng1092-103. [DOI] [PubMed] [Google Scholar]
- 25.Arthus MF, Lonergan M, Crumley MJ, et al. Report of 33 novel AVPR2 mutations and analysis of 117 families with X-linked nephrogenic diabetes insipidus. J Am Soc Nephrol. 2000;11:1044–1054. doi: 10.1681/ASN.V1161044. [DOI] [PubMed] [Google Scholar]
- 26.Dorsa DM, Majumdar LA, Petracca FM, Baskin DG, Cornett LE. Characterization and localization of 3H-arginine8-vasopressin binding to rat kidney and brain tissue. Peptides. 1983;4:699–706. doi: 10.1016/0196-9781(83)90021-9. [DOI] [PubMed] [Google Scholar]
- 27.Ostrowski NL, Lolait SJ, Bradley DJ, O’Carroll AM, Brownstein MJ, Young WS., 3rd Distribution of V1a and V2 vasopressin receptor messenger ribonucleic acids in rat liver, kidney, pituitary and brain. Endocrinology. 1992;131:533–535. doi: 10.1210/endo.131.1.1535312. [DOI] [PubMed] [Google Scholar]
- 28.Lee JW, Chou CL, Knepper MA. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol. 2015;26:2669–2677. doi: 10.1681/ASN.2014111067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ostrowski NL, Young WS, 3rd, Knepper MA, Lolait SJ. Expression of vasopressin V1a and V2 receptor messenger ribonucleic acid in the liver and kidney of embryonic, developing, and adult rats. Endocrinology. 1993;133:1849–1859. doi: 10.1210/endo.133.4.8404628. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura Y, Uchida S, Rai T, Sasaki S, Marumo F. Transcriptional regulation of aquaporin-2 water channel gene by cAMP. J Am Soc Nephrol. 1997;8:861–867. doi: 10.1681/ASN.V86861. [DOI] [PubMed] [Google Scholar]
- 31.Yasui M, Zelenin SM, Celsi G, Aperia A. Adenylate cyclase-coupled vasopressin receptor activates AQP2 promoter via a dual effect on CRE and AP1 elements. Am J Physiol. 1997;272:F443–F450. doi: 10.1152/ajprenal.1997.272.4.F443. [DOI] [PubMed] [Google Scholar]
- 32.Rieg T, Tang T, Murray F, et al. Adenylate cyclase 6 determines cAMP formation and aquaporin-2 phosphorylation and trafficking in inner medulla. J Am Soc Nephrol. 2010;21:2059–2068. doi: 10.1681/ASN.2010040409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nedvetsky PI, Tamma G, Beulshausen S, Valenti G, Rosenthal W, Klussmann E. Regulation of aquaporin-2 trafficking. Handb Exp Pharmacol. 2009:133–157. doi: 10.1007/978-3-540-79885-9_6. [DOI] [PubMed] [Google Scholar]
- 34.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Association of beta-arrestin with G protein-coupled receptors during clathrin-mediated endocytosis dictates the profile of receptor resensitization. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci U S A. 1995;92:1013–1017. doi: 10.1073/pnas.92.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishibashi K, Sasaki S, Fushimi K, et al. Molecular cloning and expression of a member of the aquaporin family with permeability to glycerol and urea in addition to water expressed at the basolateral membrane of kidney collecting duct cells. Proc Natl Acad Sci U S A. 1994;91:6269–6273. doi: 10.1073/pnas.91.14.6269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rojek A, Füchtbauer EM, Kwon TH, Frøkiaer J, Nielsen S. Severe urinary concentrating defect in renal collecting duct-selective AQP2 conditional-knockout mice. Proc Natl Acad Sci U S A. 2006;103:6037–6042. doi: 10.1073/pnas.0511324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamamoto T, Sasaki S, Fushimi K, et al. Vasopressin increases AQP-CD water channel in apical membrane of collecting duct cells in Brattleboro rats. Am J Physiol. 1995;268:C1546–C1551. doi: 10.1152/ajpcell.1995.268.6.C1546. [DOI] [PubMed] [Google Scholar]
- 39.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol. 1997;272:F817–F822. [PubMed] [Google Scholar]
- 40.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 41.Isobe K, Jung HJ, Yang CR, et al. Systems-level identification of PKA-dependent signaling in epithelial cells. Proc Natl Acad Sci U S A. 2017;114:E8875–E8884. doi: 10.1073/pnas.1709123114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Terris J, Ecelbarger CA, Nielsen S, Knepper MA. Long-term regulation of four renal aquaporins in rats. Am J Physiol. 1996;271:F414–F422. doi: 10.1152/ajprenal.1996.271.2.F414. [DOI] [PubMed] [Google Scholar]
- 43.Sandoval PC, Claxton JS, Lee JW, Saeed F, Hoffert JD, Knepper MA. Systems-level analysis reveals selective regulation of Aqp2 gene expression by vasopressin. Sci Rep. 2016;6:34863. doi: 10.1038/srep34863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hasler U, Mordasini D, Bens M, et al. Long term regulation of aquaporin-2 expression in vasopressin-responsive renal collecting duct principal cells. J Biol Chem. 2002;277:10379–10386. doi: 10.1074/jbc.M111880200. [DOI] [PubMed] [Google Scholar]
- 45.Sandoval PC, Slentz DH, Pisitkun T, Saeed F, Hoffert JD, Knepper MA. Proteome-wide measurement of protein half-lives and translation rates in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol. 2013;24:1793–1805. doi: 10.1681/ASN.2013030279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hasler U, Nielsen S, Féraille E, Martin PY. Posttranscriptional control of aquaporin-2 abundance by vasopressin in renal collecting duct principal cells. Am J Physiol Renal Physiol. 2006;290:F177–F187. doi: 10.1152/ajprenal.00056.2005. [DOI] [PubMed] [Google Scholar]
- 47.Lee YJ, Lee JE, Choi HJ, et al. E3 ubiquitin-protein ligases in rat kidney collecting duct: response to vasopressin stimulation and withdrawal. Am J Physiol Renal Physiol. 2011;301:F883–F896. doi: 10.1152/ajprenal.00117.2011. [DOI] [PubMed] [Google Scholar]
- 48.Medvar B, Raghuram V, Pisitkun T, Sarkar A, Knepper MA. Comprehensive database of human E3 ubiquitin ligases: application to aquaporin-2 regulation. Physiol Genomics. 2016;48:502–512. doi: 10.1152/physiolgenomics.00031.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu Q, Moeller HB, Stevens DA, et al. CHIP regulates aquaporin-2 quality control and body water homeostasis. J Am Soc Nephrol. 2018;29:936–948. doi: 10.1681/ASN.2017050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centrone M, Ranieri M, Di Mise A, et al. AQP2 abundance is regulated by the E3-ligase CHIP via HSP70. Cell Physiol Biochem. 2017;44:515–531. doi: 10.1159/000485088. [DOI] [PubMed] [Google Scholar]
- 51.Trimpert C, Wesche D, de Groot T, et al. NDFIP allows NEDD4/NEDD4L-induced AQP2 ubiquitination and degradation. PLoS One. 2017;12:e0183774. doi: 10.1371/journal.pone.0183774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasler U, Leroy V, Martin PY, Féraille E. Aquaporin-2 abundance in the renal collecting duct: new insights from cultured cell models. Am J Physiol Renal Physiol. 2009;297:F10–F18. doi: 10.1152/ajprenal.00053.2009. [DOI] [PubMed] [Google Scholar]
- 53.Kim JE, Jung HJ, Lee YJ, Kwon TH. Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am J Physiol Renal Physiol. 2015;308:F749–F764. doi: 10.1152/ajprenal.00334.2014. [DOI] [PubMed] [Google Scholar]
- 54.Ranieri M, Zahedi K, Tamma G, et al. CaSR signaling down-regulates AQP2 expression via a novel microRNA pathway in pendrin and NaCl cotransporter knockout mice. FASEB J. 2018;32:2148–2159. doi: 10.1096/fj.201700412RR. [DOI] [PubMed] [Google Scholar]
- 55.Bijkerk R, Trimpert C, van Solingen C, et al. MicroRNA-132 controls water homeostasis through regulating MECP2-mediated vasopressin synthesis. Am J Physiol Renal Physiol. 2018;315:F1129–F1138. doi: 10.1152/ajprenal.00087.2018. [DOI] [PubMed] [Google Scholar]
- 56.Bockenhauer D, Bichet DG. Pathophysiology, diagnosis and management of nephrogenic diabetes insipidus. Nat Rev Nephrol. 2015;11:576–588. doi: 10.1038/nrneph.2015.89. [DOI] [PubMed] [Google Scholar]
- 57.Milano S, Carmosino M, Gerbino A, Svelto M, Procino G. Hereditary nephrogenic diabetes insipidus: pathophysiology and possible treatment. An update. Int J Mol Sci. 2017;18:2385. doi: 10.3390/ijms18112385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujiwara TM, Morgan K, Bichet DG. Molecular biology of diabetes insipidus. Annu Rev Med. 1995;46:331–343. doi: 10.1146/annurev.med.46.1.331. [DOI] [PubMed] [Google Scholar]
- 59.Deen PM, Verdijk MA, Knoers NV, et al. Requirement of human renal water channel aquaporin-2 for vasopressin-dependent concentration of urine. Science. 1994;264:92–95. doi: 10.1126/science.8140421. [DOI] [PubMed] [Google Scholar]
- 60.Sohara E, Rai T, Yang SS, et al. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc Natl Acad Sci U S A. 2006;103:14217–14222. doi: 10.1073/pnas.0602331103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bichet DG. Vasopressin receptor mutations in nephrogenic diabetes insipidus. Semin Nephrol. 2008;28:245–251. doi: 10.1016/j.semnephrol.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 62.Babey M, Kopp P, Robertson GL. Familial forms of diabetes insipidus: clinical and molecular characteristics. Nat Rev Endocrinol. 2011;7:701–714. doi: 10.1038/nrendo.2011.100. [DOI] [PubMed] [Google Scholar]
- 63.Baylis PH. Osmoregulation and control of vasopressin secretion in healthy humans. Am J Physiol. 1987;253:R671–R678. doi: 10.1152/ajpregu.1987.253.5.R671. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen S, DiGiovanni SR, Christensen EI, Knepper MA, Harris HW. Cellular and subcellular immunolocalization of vasopressin-regulated water channel in rat kidney. Proc Natl Acad Sci U S A. 1993;90:11663–11667. doi: 10.1073/pnas.90.24.11663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yasui M, Marples D, Belusa R, et al. Development of urinary concentrating capacity: role of aquaporin-2. Am J Physiol. 1996;271:F461–F468. doi: 10.1152/ajprenal.1996.271.2.F461. [DOI] [PubMed] [Google Scholar]
- 66.Ecelbarger CA, Nielsen S, Olson BR, et al. Role of renal aquaporins in escape from vasopressin-induced antidiuresis in rat. J Clin Invest. 1997;99:1852–1863. doi: 10.1172/JCI119352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Klussmann E, Maric K, Wiesner B, Beyermann M, Rosenthal W. Protein kinase A anchoring proteins are required for vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem. 1999;274:4934–4938. doi: 10.1074/jbc.274.8.4934. [DOI] [PubMed] [Google Scholar]
- 68.Stefan E, Wiesner B, Baillie GS, et al. Compartmentalization of cAMP-dependent signaling by phosphodiesterase-4D is involved in the regulation of vasopressin-mediated water reabsorption in renal principal cells. J Am Soc Nephrol. 2007;18:199–212. doi: 10.1681/ASN.2006020132. [DOI] [PubMed] [Google Scholar]
- 69.Hozawa S, Holtzman EJ, Ausiello DA. cAMP motifs regulating transcription in the aquaporin 2 gene. Am J Physiol. 1996;270:C1695–C1702. doi: 10.1152/ajpcell.1996.270.6.C1695. [DOI] [PubMed] [Google Scholar]
- 70.Kim HY, Choi HJ, Lim JS, et al. Emerging role of Akt substrate protein AS160 in the regulation of AQP2 translocation. Am J Physiol Renal Physiol. 2011;301:F151–F161. doi: 10.1152/ajprenal.00519.2010. [DOI] [PubMed] [Google Scholar]
- 71.Lee YJ, Song IK, Jang KJ, et al. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT1 receptor. Am J Physiol Renal Physiol. 2007;292:F340–F350. doi: 10.1152/ajprenal.00090.2006. [DOI] [PubMed] [Google Scholar]
- 72.Li C, Wang W, Rivard CJ, Lanaspa MA, Summer S, Schrier RW. Molecular mechanisms of angiotensin II stimulation on aquaporin-2 expression and trafficking. Am J Physiol Renal Physiol. 2011;300:F1255–F1261. doi: 10.1152/ajprenal.00469.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Olesen ET, Moeller HB, Assentoft M, MacAulay N, Fenton RA. The vasopressin type 2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP-independent pathway. Am J Physiol Renal Physiol. 2016;311:F935–F944. doi: 10.1152/ajprenal.00559.2015. [DOI] [PubMed] [Google Scholar]
- 74.Rao R, Patel S, Hao C, Woodgett J, Harris R. GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J Am Soc Nephrol. 2010;21:428–437. doi: 10.1681/ASN.2009060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pisitkun T, Jacob V, Schleicher SM, Chou CL, Yu MJ, Knepper MA. Akt and ERK1/2 pathways are components of the vasopressin signaling network in rat native IMCD. Am J Physiol Renal Physiol. 2008;295:F1030–F1043. doi: 10.1152/ajprenal.90339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Procino G, Gerbino A, Milano S, et al. Rosiglitazone promotes AQP2 plasma membrane expression in renal cells via a Ca-dependent/cAMP-independent mechanism. Cell Physiol Biochem. 2015;35:1070–1085. doi: 10.1159/000373933. [DOI] [PubMed] [Google Scholar]
- 77.Li S, Qiu M, Kong Y, et al. Bile acid G protein-coupled membrane receptor TGR5 modulates aquaporin 2-mediated water homeostasis. J Am Soc Nephrol. 2018;29:2658–2670. doi: 10.1681/ASN.2018030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kortenoeven ML, Trimpert C, van den Brand M, Li Y, Wetzels JF, Deen PM. In mpkCCD cells, long-term regulation of aquaporin-2 by vasopressin occurs independent of protein kinase A and CREB but may involve Epac. Am J Physiol Renal Physiol. 2012;302:F1395–F1401. doi: 10.1152/ajprenal.00376.2011. [DOI] [PubMed] [Google Scholar]
- 79.de Rooij J, Zwartkruis FJ, Verheijen MH, et al. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 80.Ong CT, Corces VG. Enhancer function: new insights into the regulation of tissue-specific gene expression. Nat Rev Genet. 2011;12:283–293. doi: 10.1038/nrg2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jung HJ, Raghuram V, Lee JW, Knepper MA. Genome-wide mapping of DNA accessibility and binding sites for CREB and C/EBPβ in vasopressin-sensitive collecting duct cells. J Am Soc Nephrol. 2018;29:1490–1500. doi: 10.1681/ASN.2017050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang J, Zibetti C, Shang P, et al. ATAC-Seq analysis reveals a widespread decrease of chromatin accessibility in age-related macular degeneration. Nat Commun. 2018;9:1364. doi: 10.1038/s41467-018-03856-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spitz F, Furlong EE. Transcription factors: from enhancer binding to developmental control. Nat Rev Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- 84.Hnisz D, Day DS, Young RA. Insulated neighborhoods: structural and functional units of Mammalian gene control. Cell. 2016;167:1188–1200. doi: 10.1016/j.cell.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shlyueva D, Stampfel G, Stark A. Transcriptional enhancers: from properties to genome-wide predictions. Nat Rev Genet. 2014;15:272–286. doi: 10.1038/nrg3682. [DOI] [PubMed] [Google Scholar]
- 86.Yu MJ, Miller RL, Uawithya P, et al. Systems-level analysis of cell-specific AQP2 gene expression in renal collecting duct. Proc Natl Acad Sci U S A. 2009;106:2441–2446. doi: 10.1073/pnas.0813002106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schenk LK, Bolger SJ, Luginbuhl K, et al. Quantitative proteomics identifies vasopressin-responsive nuclear proteins in collecting duct cells. J Am Soc Nephrol. 2012;23:1008–1018. doi: 10.1681/ASN.2011070738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Park EJ, Lim JS, Jung HJ, Kim E, Han KH, Kwon TH. The role of 70-kDa heat shock protein in dDAVP-induced AQP2 trafficking in kidney collecting duct cells. Am J Physiol Renal Physiol. 2013;304:F958–F971. doi: 10.1152/ajprenal.00469.2012. [DOI] [PubMed] [Google Scholar]
- 89.Hwang JR, Chou CL, Medvar B, Knepper MA, Jung HJ. Identification of β-catenin-interacting proteins in nuclear fractions of native rat collecting duct cells. Am J Physiol Renal Physiol. 2017;313:F30–F46. doi: 10.1152/ajprenal.00054.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grassmeyer J, Mukherjee M, deRiso J, et al. Elf5 is a principal cell lineage specific transcription factor in the kidney that contributes to Aqp2 and Avpr2 gene expression. Dev Biol. 2017;424:77–89. doi: 10.1016/j.ydbio.2017.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu L, Moriguchi T, Souma T, et al. GATA2 regulates body water homeostasis through maintaining aquaporin 2 expression in renal collecting ducts. Mol Cell Biol. 2014;34:1929–1941. doi: 10.1128/MCB.01659-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang X, Huang S, Gao M, et al. Farnesoid X receptor (FXR) gene deficiency impairs urine concentration in mice. Proc Natl Acad Sci U S A. 2014;111:2277–2282. doi: 10.1073/pnas.1323977111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hasler U, Vinciguerra M, Vandewalle A, Martin PY, Féraille E. Dual effects of hypertonicity on aquaporin-2 expression in cultured renal collecting duct principal cells. J Am Soc Nephrol. 2005;16:1571–1582. doi: 10.1681/ASN.2004110930. [DOI] [PubMed] [Google Scholar]
- 94.Kortenoeven ML, van den Brand M, Wetzels JF, Deen PM. Hypotonicity-induced reduction of aquaporin-2 transcription in mpkCCD cells is independent of the tonicity responsive element, vasopressin, and cAMP. J Biol Chem. 2011;286:13002–13010. doi: 10.1074/jbc.M110.207878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li SZ, McDill BW, Kovach PA, et al. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol. 2007;292:C1606–C1616. doi: 10.1152/ajpcell.00588.2005. [DOI] [PubMed] [Google Scholar]
- 96.Hasler U, Leroy V, Jeon US, et al. NF-kappaB modulates aquaporin-2 transcription in renal collecting duct principal cells. J Biol Chem. 2008;283:28095–28105. doi: 10.1074/jbc.M708350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Olesen ET, de Seigneux S, Wang G, et al. Rapid and segmental specific dysregulation of AQP2, S256-pAQP2 and renal sodium transporters in rats with LPS-induced endotoxaemia. Nephrol Dial Transplant. 2009;24:2338–2349. doi: 10.1093/ndt/gfp011. [DOI] [PubMed] [Google Scholar]
- 98.Furey TS. ChIP-seq and beyond: new and improved methodologies to detect and characterize protein-DNA interactions. Nat Rev Genet. 2012;13:840–852. doi: 10.1038/nrg3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms? Genes Dev. 2004;18:2046–2059. doi: 10.1101/gad.1214604. [DOI] [PubMed] [Google Scholar]
- 101.Weake VM, Workman JL. Histone ubiquitination: triggering gene activity. Mol Cell. 2008;29:653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 102.Dancy BM, Cole PA. Protein lysine acetylation by p300/ CBP. Chem Rev. 2015;115:2419–2452. doi: 10.1021/cr500452k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hyndman KA, Knepper MA. Dynamic regulation of lysine acetylation: the balance between acetyltransferase and deacetylase activities. Am J Physiol Renal Physiol. 2017;313:F842–F846. doi: 10.1152/ajprenal.00313.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hecht A, Vleminckx K, Stemmler MP, van Roy F, Kemler R. The p300/CBP acetyltransferases function as transcriptional coactivators of beta-catenin in vertebrates. EMBO J. 2000;19:1839–1850. doi: 10.1093/emboj/19.8.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bansal AD, Hoffert JD, Pisitkun T, et al. Phosphoproteomic profiling reveals vasopressin-regulated phosphorylation sites in collecting duct. J Am Soc Nephrol. 2010;21:303–315. doi: 10.1681/ASN.2009070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA. Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteomics. 2012;11 doi: 10.1074/mcp.M111.014613. M111.014613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jung HJ, Kim SY, Choi HJ, et al. Tankyrase-mediated β-catenin activity regulates vasopressin-induced AQP2 expression in kidney collecting duct mpkCCDc14 cells. Am J Physiol Renal Physiol. 2015;308:F473–F486. doi: 10.1152/ajprenal.00052.2014. [DOI] [PubMed] [Google Scholar]
- 108.Yu Z, Kong Q, Kone BC. Aldosterone reprograms promoter methylation to regulate αENaC transcription in the collecting duct. Am J Physiol Renal Physiol. 2013;305:F1006–F1013. doi: 10.1152/ajprenal.00407.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marumo T, Yagi S, Kawarazaki W, et al. Diabetes induces aberrant DNA methylation in the proximal tubules of the kidney. J Am Soc Nephrol. 2015;26:2388–2397. doi: 10.1681/ASN.2014070665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhao Y, Ding C, Xue W, et al. Genome-wide DNA methylation analysis in renal ischemia reperfusion injury. Gene. 2017;610:32–43. doi: 10.1016/j.gene.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 111.Liu Y, Liu P, Yang C, Cowley AW, Jr, Liang M. Base-resolution maps of 5-methylcytosine and 5-hydroxymethylcytosine in Dahl S rats: effect of salt and genomic sequence. Hypertension. 2014;63:827–838. doi: 10.1161/HYPERTENSIONAHA.113.02637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wesche D, Deen PM, Knoers NV. Congenital nephrogenic diabetes insipidus: the current state of affairs. Pediatr Nephrol. 2012;27:2183–2204. doi: 10.1007/s00467-012-2118-8. [DOI] [PubMed] [Google Scholar]
- 113.Birnbaumer M. Vasopressin receptor mutations and nephrogenic diabetes insipidus. Arch Med Res. 1999;30:465–474. doi: 10.1016/S0188-4409(99)00063-6. [DOI] [PubMed] [Google Scholar]
- 114.Spanakis E, Milord E, Gragnoli C. AVPR2 variants and mutations in nephrogenic diabetes insipidus: review and missense mutation significance. J Cell Physiol. 2008;217:605–617. doi: 10.1002/jcp.21552. [DOI] [PubMed] [Google Scholar]
- 115.Robben JH, Knoers NV, Deen PM. Characterization of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus in a polarized cell model. Am J Physiol Renal Physiol. 2005;289:F265–F272. doi: 10.1152/ajprenal.00404.2004. [DOI] [PubMed] [Google Scholar]
- 116.Morello JP, Salahpour A, Laperrière A, et al. Pharmacological chaperones rescue cell-surface expression and function of misfolded V2 vasopressin receptor mutants. J Clin Invest. 2000;105:887–895. doi: 10.1172/JCI8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robben JH, Deen PM. Pharmacological chaperones in nephrogenic diabetes insipidus: possibilities for clinical application. BioDrugs. 2007;21:157–166. doi: 10.2165/00063030-200721030-00003. [DOI] [PubMed] [Google Scholar]
- 118.Barak LS, Oakley RH, Laporte SA, Caron MG. Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci U S A. 2001;98:93–98. doi: 10.1073/pnas.98.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robben JH, Sze M, Knoers NV, Deen PM. Functional rescue of vasopressin V2 receptor mutants in MDCK cells by pharmacochaperones: relevance to therapy of nephrogenic diabetes insipidus. Am J Physiol Renal Physiol. 2007;292:F253–F260. doi: 10.1152/ajprenal.00247.2006. [DOI] [PubMed] [Google Scholar]
- 120.Wüller S, Wiesner B, Löffler A, et al. Pharmacochaperones post-translationally enhance cell surface expression by increasing conformational stability of wild-type and mutant vasopressin V2 receptors. J Biol Chem. 2004;279:47254–47263. doi: 10.1074/jbc.M408154200. [DOI] [PubMed] [Google Scholar]
- 121.Serradeil-Le Gal C, Wagnon J, Garcia C, et al. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bernier V, Lagacé M, Lonergan M, Arthus MF, Bichet DG, Bouvier M. Functional rescue of the constitutively internalized V2 vasopressin receptor mutant R137H by the pharmacological chaperone action of SR49059. Mol Endocrinol. 2004;18:2074–2084. doi: 10.1210/me.2004-0080. [DOI] [PubMed] [Google Scholar]
- 123.Los EL, Deen PM, Robben JH. Potential of nonpeptide (ant)agonists to rescue vasopressin V2 receptor mutants for the treatment of X-linked nephrogenic diabetes insipidus. J Neuroendocrinol. 2010;22:393–399. doi: 10.1111/j.1365-2826.2010.01983.x. [DOI] [PubMed] [Google Scholar]
- 124.Robben JH, Kortenoeven ML, Sze M, et al. Intracellular activation of vasopressin V2 receptor mutants in nephrogenic diabetes insipidus by nonpeptide agonists. Proc Natl Acad Sci U S A. 2009;106:12195–12200. doi: 10.1073/pnas.0900130106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jean-Alphonse F, Perkovska S, Frantz MC, et al. Biased agonist pharmacochaperones of the AVP V2 receptor may treat congenital nephrogenic diabetes insipidus. J Am Soc Nephrol. 2009;20:2190–2203. doi: 10.1681/ASN.2008121289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Procino G, Carmosino M, Milano S, et al. β3 adrenergic receptor in the kidney may be a new player in sympathetic regulation of renal function. Kidney Int. 2016;90:555–567. doi: 10.1016/j.kint.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chu JY, Chung SC, Lam AK, Tam S, Chung SK, Chow BK. Phenotypes developed in secretin receptor-null mice indicated a role for secretin in regulating renal water reabsorption. Mol Cell Biol. 2007;27:2499–2511. doi: 10.1128/MCB.01088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Charlton CG, Quirion R, Handelmann GE, et al. Secretin receptors in the rat kidney: adenylate cyclase activation and renal effects. Peptides. 1986;7:865–871. doi: 10.1016/0196-9781(86)90107-5. [DOI] [PubMed] [Google Scholar]
- 129.Li JH, Chou CL, Li B, et al. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J Clin Invest. 2009;119:3115–3126. doi: 10.1172/JCI39680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Olesen ET, Rützler MR, Moeller HB, Praetorius HA, Fenton RA. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc Natl Acad Sci U S A. 2011;108:12949–12954. doi: 10.1073/pnas.1104691108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Carney S, Morgan T, Ray C, Thompson L. Effect of calcitonin on urine concentration in the rat. Am J Physiol. 1983;244:F432–F435. doi: 10.1152/ajprenal.1983.244.4.F432. [DOI] [PubMed] [Google Scholar]
- 132.Bouley R, Lu HA, Nunes P, et al. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J Am Soc Nephrol. 2011;22:59–72. doi: 10.1681/ASN.2009121267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Procino G, Milano S, Carmosino M, et al. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int. 2014;86:127–138. doi: 10.1038/ki.2014.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fukuhara S, Sakurai A, Sano H, et al. Cyclic AMP potentiates vascular endothelial cadherin-mediated cell-cell contact to enhance endothelial barrier function through an Epac-Rap1 signaling pathway. Mol Cell Biol. 2005;25:136–146. doi: 10.1128/MCB.25.1.136-146.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wang W, Li C, Kwon TH, Knepper MA, Frøkiaer J, Nielsen S. AQP3, p-AQP2, and AQP2 expression is reduced in polyuric rats with hypercalcemia: prevention by cAMP-PDE inhibitors. Am J Physiol Renal Physiol. 2002;283:F1313–F1325. doi: 10.1152/ajprenal.00040.2002. [DOI] [PubMed] [Google Scholar]
- 136.Bouley R, Pastor-Soler N, Cohen O, McLaughlin M, Breton S, Brown D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra) Am J Physiol Renal Physiol. 2005;288:F1103–F1112. doi: 10.1152/ajprenal.00337.2004. [DOI] [PubMed] [Google Scholar]
- 137.Sanches TR, Volpini RA, Massola Shimizu MH, et al. Silde-nafil reduces polyuria in rats with lithium-induced NDI. Am J Physiol Renal Physiol. 2012;302:F216–F225. doi: 10.1152/ajprenal.00439.2010. [DOI] [PubMed] [Google Scholar]
- 138.Ando F, Sohara E, Morimoto T, et al. Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway. Nat Commun. 2016;7:13636. doi: 10.1038/ncomms13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Cheung PW, Nomura N, Nair AV, et al. EGF receptor inhibition by erlotinib increases aquaporin 2-mediated renal water reabsorption. J Am Soc Nephrol. 2016;27:3105–3116. doi: 10.1681/ASN.2015080903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tingskov SJ, Hu S, Frøkiær J, Kwon TH, Wang W, Nør-regaard R. Tamoxifen attenuates development of lithium-induced nephrogenic diabetes insipidus in rats. Am J Physiol Renal Physiol. 2018;314:F1020–F1025. doi: 10.1152/ajprenal.00604.2017. [DOI] [PubMed] [Google Scholar]
- 141.Efe O, Klein JD, LaRocque LM, Ren H, Sands JM. Metformin improves urine concentration in rodents with nephrogenic diabetes insipidus. JCI Insight. 2016;1:e88409. doi: 10.1172/jci.insight.88409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li W, Zhang Y, Bouley R, et al. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am J Physiol Renal Physiol. 2011;301:F309–F318. doi: 10.1152/ajprenal.00001.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Procino G, Barbieri C, Carmosino M, et al. Fluvastatin modulates renal water reabsorption in vivo through increased AQP2 availability at the apical plasma membrane of collecting duct cells. Pflugers Arch. 2011;462:753–766. doi: 10.1007/s00424-011-1007-5. [DOI] [PubMed] [Google Scholar]