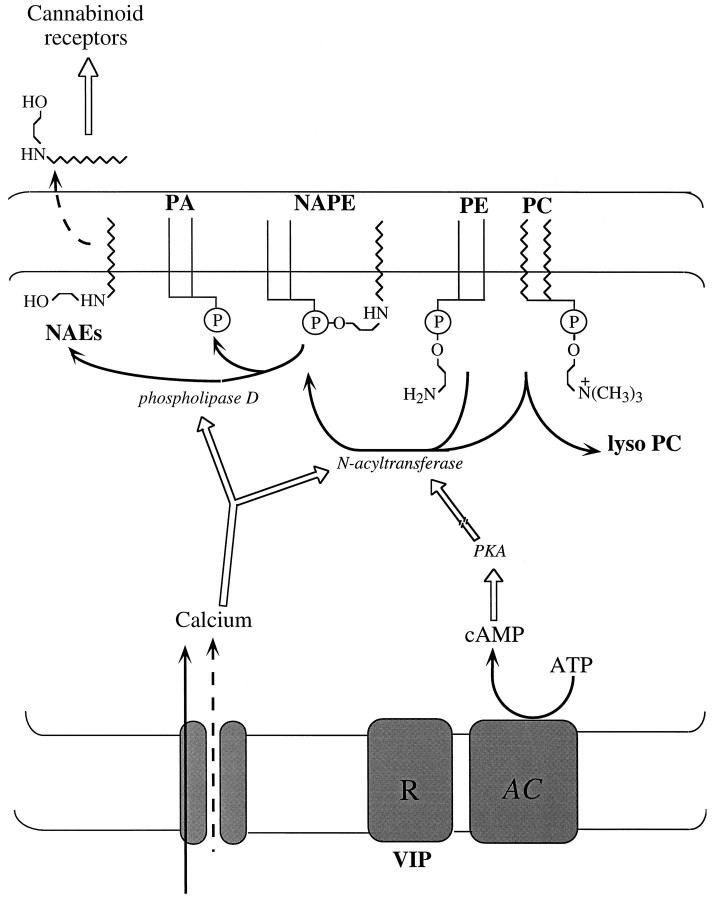
Fig. 6.
Model of biosynthesis and regulation of the endogenous cannabinoid precursor NAPE in rat cortical neurons. [Ca2+]i rises produced by neuronal depolarization may stimulate an N-acyltransferase activity that catalyzes the intermolecular transfer of a fatty acyl group from a glycerophospholipid [e.g., phosphatidylcholine (PC)], to the ethanolamine moiety of phosphatidylethanolamine (PE), forming NAPE and lysophospholipid (e.g., lyso PC). Neuromodulators (e.g., VIP) may enhance Ca2+-dependent NAPE biosynthesis by activating a membrane receptor (R) coupled to the activation of adenylyl cyclase (AC) and to the subsequent stimulation of cAMP-dependent protein kinase (PKA) activity. The broken arrow indicates that the molecular target of PKA leading to enhanced NAPE biosynthesis remains to be determined. NAPE is composed of several molecular species, differing in the fatty acyl group linked to the ethanolamine moiety (Schmid et al., 1990; Cadas et al., 1996). Therefore, cleavage of NAPE by a D-type phosphodiesterase activity [phospholipase D (PLD)] may give rise to multipleN-acylethanolamines (NAEs), including anandamide (which activates cannabinoid CB1-type receptors) andN-palmitoylethanolamine (which activates CB2-type receptors in certain cell types). Although cannabimimetic NAEs may be recovered in the extracellular fluid of stimulated neurons in culture (Di Marzo et al., 1994; Hansen et al., 1995), the mechanism of extrusion of these lipids (indicated schematically as a broken arrow) is still unknown.