Fig. 4.
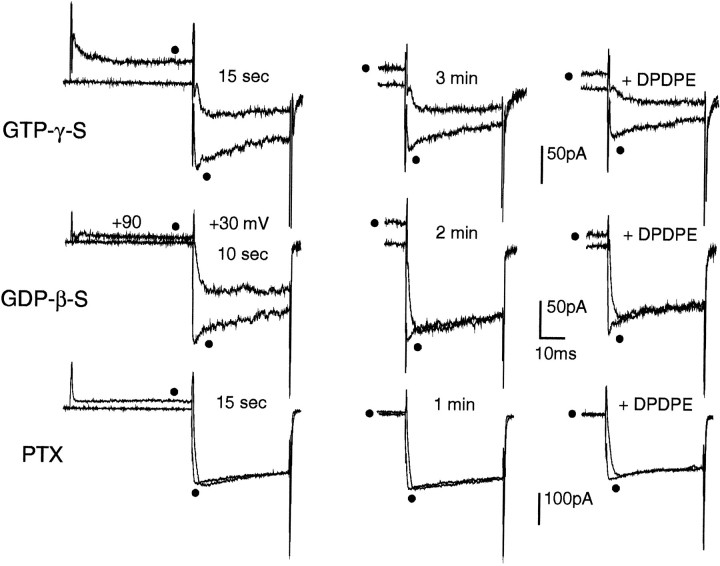
Ba2+ current inhibition by opioids is mediated by PTX-sensitive G-proteins. Top, Intracellular GTPγS (100 μm) mimics and preserves the inhibitory action of DPDPE in a GLC8 cell already inhibited tonically from the start of the whole-cell recordings (t = 15 sec) (left). The recordings consisted of a double-pulse protocol similar to that in Figure 3B, which allows the determination sequentially of the degree of inhibition and facilitation (filled circles) either present at control (left and middle panels) or induced by the opioid (right panels). After 3 min of internal perfusion, GTPγS preserved both the tonic (middle) and the 0.5 μm DPDPE-induced (right) inhibition. The more pronounced kinetics slowing induced by DPDPE was preserved after washout of the agonist, suggesting that GTPγS makes irreversible the usually reversible effect of DPDPE. Middle, Intracellular GDPβS (500 μm) rapidly removed both tonic and DPDPE-induced inhibition. The GLC8 cell was inhibited tonically at the start of internal dialysis (t = 10 sec) (left). After 2 min of internal perfusion with GDPβS, the inhibition was abolished completely (middle), and subsequent application of DPDPE (0.5 μm) had no effects (right). Bottom, Cell preincubation with PTX (100 ng/ml, 15 hr) prevented fully the Ba2+ current inhibition by opioids. The PTX-treated cell exhibited no sign of tonic inhibition after 15 sec (left) and 1 min (middle) from the beginning of intracellular perfusion and was insensitive to 0.5 μm DPDPE (right). Note the total absence of voltage-dependent facilitation of Ba2+currents in this PTX pretreated cell. The slight decrease of current amplitude observed during exposure to DPDPE (right) was not reversible and was probably attributable to partial channel rundown.