Abstract
The inactivation kinetics of the Na+ current of the weakly electric fish Sternopygus are modified by treatment with androgens. To determine whether phosphorylation could play a role in this effect, we examined whether activation of protein kinase A by 8 bromo cyclic AMP (8 Br cAMP) altered voltage-dependent properties of the current. Using a two-electrode voltage-clamp procedure, we found no effect of 8 Br cAMP on inactivation kinetics or other voltage-dependent properties of the Na+current of the electrocytes. However, treatment with 8 Br cAMP did produce a dose-dependent increase in the Na+current compared with saline controls: 17.6% at 100 μm, 42.4% at 1 mm, and 43.1% at 5 mm. This effect was blocked by 30 μm H89, a PKA inhibitor, indicating that the observed effect was attributable to 8 Br cAMP activation of PKA. We conclude that androgen-induced changes in Na+current inactivation are not mediated by PKA and suggest that PKA-mediated increases in Na+ current underlie increases in the amplitude of the electric organ discharge observed in social interactions or with changes in water conductance.
Keywords: protein kinase A, sodium channel, phosphorylation, cyclic adenosine monophosphate, 8 Br cAMP, electric fish, electric organ
Voltage-dependent Na+channels are responsible for the depolarization of nerve and muscle cells that initiates the action potential (AP). Despite the all or none nature of the AP, characteristics such as duration, refractory period, or amplitude may vary, depending, for example, on cell type or developmental stage. Although K+ channel diversity is often responsible for shaping the unique characteristics of APs in specific cell types, it has become increasingly clear that diversity in and modulation of Na+ channels also can underlie AP variation.
This can be seen clearly in the AP of the electric organ (EO) of the weakly electric fish Sternopygus. Like other electric fish,Sternopygus generates an electric field from the summed APs of the electrocytes comprising its EO (Bennett, 1961, 1971), which is used in electrolocation and in social communication (Bullock and Heiligenberg, 1986). Within the species-specific range of EO discharge (EOD) frequencies (50–200 Hz), individual Sternopygusdisplay an individually distinct and sexually dimorphic EOD, with sexually mature males displaying lower EOD frequencies (50–90 Hz) and females discharging at higher frequencies (110–200 Hz) (Hopkins, 1972,1974; Meyer, 1983; Zakon et al., 1991).
The EOD waveform is determined both by the firing frequency of the medullary pacemaker nucleus, as well as the AP duration of the electrocytes. AP duration varies with EOD frequency in a graded manner such that electrocytes in fish that generate the lowest frequency EODs have the longest duration APs, whereas electrocytes from fish with high-frequency EODs make short-duration APs (Mills and Zakon, 1987,1991). The fast inactivation kinetics of the Na+current vary systematically with AP duration, and this variation is the basis for the individual differences in AP duration (Ferrari et al., 1995). Androgen strongly modulates EO output; dihydrotestosterone (DHT) implants lower the EOD frequency, broaden the AP duration (Mills and Zakon, 1987, 1991), and slow the Na+ current inactivation time constant (Ferrari et al., 1995).
Several mechanisms could give rise to individual variations in and androgen-dependent modulation of Na+ channel kinetics. Different isoforms of the Na+ channel have been described (Hille, 1992), which may vary kinetically (Kallen et al., 1990; Elliott and Elliott, 1993; Ogata and Tatebayashi, 1993;Rizzo et al., 1994). In some tissues, association of β subunits with the main (α) subunit influences channel kinetics (Isom et al., 1992). Finally, post-translational modification of Na+channels by phosphorylation can regulate Na+channel function (Numann et al., 1991; Li et al., 1993).
In this study, we investigated the role of phosphorylation via activation of protein kinase A (PKA) on Na+channel kinetics and amplitude. We focused on PKA because work on the phylogenetically related eel Electrophorus electricusindicates that PKA, but not protein kinase C (PKC), phosphorylates the eel EO Na+ channel, resulting in physiologically significant modulation of the channel (Emerick and Agnew, 1989; Emerick et al., 1993).
MATERIALS AND METHODS
Animals. Sternopygus macrurus were obtained commercially and maintained in aquaria in controlled temperature chambers. Immediately before electrocyte recording, external recordings of the whole animal’s EOD frequency were made in the home aquarium.
Tissue preparation. The Sternopygus EO preparation has been described previously (Ferrari and Zakon, 1993;Ferrari et al., 1995). A small (1.5–2.0 cm) section of the tail was removed and placed in a simplified Hickman’s saline containing (in mm): 114 NaCl, 2 KCl, 4 CaCl2, 2 MgCl2, 5 HEPES, 3 glucose, pH = 7.2. Curare (d-tubocurarine chloride, 5 mg/l, Sigma, St. Louis, MO) was added to prevent spontaneous contractions of the small muscle fibers in the tail. The skin was removed to expose the electrocytes for intracellular recording, and the tissue was pinned into a SYLGARD recording chamber. Recordings were made at room temperature (23 ± 1°C).
Voltage clamp. The voltage-clamp procedure forSternopygus has been described previously (Ferrari and Zakon, 1993; Ferrari et al., 1995). Two microelectrodes were used to voltage-clamp the electrocytes (Axoclamp 2-A amplifier, TL-1 DMA interface and pClamp software, Axon Instruments, Foster City, CA; Lab Master DMA boards, Scientific Solutions, Solon, OH; and Dell 325D computer, Austin, TX). Microelectrodes were pulled from thin-wall filament glass (No. 6160, A-M Systems, Everett, WA) on a Flaming–Brown model P-80/PC and had resistances of 0.5–2.5 MΩ when filled with 3 m KCl. A grounded shield was placed between the two electrodes and lowered as close to the bath surface as possible. The saline level was also adjusted so as to just cover the tissue surface, which, in conjunction with the grounded shield, served to reduce coupling capacitance between the electrodes. The two microelectrodes were placed in the posterior, active end of superficial electrocytes. The ground electrode was a chlorided silver wire inserted into a plastic tube filled with 3% agar in 3 mKCl.
As described previously (Ferrari and Zakon, 1993), to achieve a good space clamp, we blocked the electrocyte’s chloride leakage by replacing the NaCl with Na+ methyl sulfate in the recording saline containing (in mm): 110 Na+ methyl sulfate, 2 KCl, 4 CaCl2, 1 MgCl2, 5 HEPES, 3 glucose, and 5 mg/l curare, pH = 7.2. In addition, 40 mm tetraethylammonium and 2 mm CsCl2 were added to block the electrocyte K+ currents. The Na+ current was isolated for analysis by subtracting the linear leakage from the Na+current by using a depolarizing prepulse to inactivate the Na+ current after K+currents were blocked pharmacologically. Criteria for a well clamped Na+ current were as described (Ferrari et al., 1995).
Pulse protocols to determine current activation, fast inactivation, steady-state inactivation, reversal potential, peak current, and refractory behavior were generated by the pClamp software and delivered to the Axoclamp 2-A amplifier via the TL-1 DMA interface. During these acquisition episodes, the membrane current was sampled at 20 kHz. Output bandwidth filter setting for the 3 db cutoff frequency was 10 kHz.
PKA experiments. Most of the fish in this study had midrange EOD frequencies (sex not determined). To determine whether activation of the PKA pathway altered Na+ channel properties in Sternopygus, the membrane-permeant cyclic adenosine monophosphate (cAMP) analog 8, bromo cAMP (8 Br cAMP) (Lot 73H7804, Sigma, St. Louis, MO) was used to activate PKA. The 8 Br cAMP was dissolved in recording saline immediately before use at concentrations of 100 μm, 1 mm, or 5 mm. After a good voltage clamp was achieved in regular recording saline, baseline recordings were made. The solution in the recording dish was then replaced by ∼10 ml of additional saline (saline control), saline-containing cAMP, or saline-containing 8 Br cAMP. The solution changes required ∼6–8 min to complete. Immediately after the solution change, an initial recording in the new saline was made and was designated as time “0.” Subsequent recordings were made at 5 or 10 min intervals.
To confirm that the 8 Br cAMP was acting via the PKA pathway, we added the PKA inhibitor H89 (lots 631393 and 827093, Calbiochem, La Jolla, CA) to the recording saline in the final set of experiments. H89 has been shown to be a potent and specific inhibitor of PKA (Chijiwa et al., 1990) which, when added to cell cultures at doses of 10–33 μm, inhibits PKA specifically with no effect on responses evoked by stimulation of PKC (Chijiwa et al., 1990; Ginty et al., 1991; Geilen et al., 1992; Takuma and Ichida, 1994). The H89 was dissolved in 100% dimethylsulfoxide (DMSO) and added to the recording saline at a final concentration of 30 μm H89 and 1% DMSO. The tissue was pretreated with recording saline containing 30 μm H89 for at least 20 min. At the end of the pretreatment period, a baseline recording was taken and then the saline was replaced with recording saline containing 1 mm 8 Br cAMP, as well as 30 μm H89. Recording intervals were the same as in saline with 8 Br cAMP alone. Controls had DMSO added to the recording saline to a final solution of 1%.
RESULTS
Effects of 8 Br cAMP on voltage-dependent parameters of theSternopygus EO Na+ current
In many cells treated with 8 Br cAMP, a slight increase in Na+ current magnitude could be seen at the earliest time recorded, i.e., as soon as we completed the solution change and began voltage-clamp recording, defined as time “0.” By 20 min after the solution change to recording saline with 8 Br cAMP, a marked increase in the Na+ current magnitude was observed (Fig. 1). However, we observed no changes between Na+ current inactivation kinetics (τh) recorded in saline and those recorded in 8 Br cAMP. In control as well as 8 Br cAMP-treated cells, we observed a slight transient decrease in τh at time “0” followed by a slight gradual increase in τhover the time course of the experiment. At 20 min after solution change, mean percent changes in τh were: NaMS 1.375% ± 14.463 (SD), n = 4; 100 μm 8 Br cAMP 2.325 ± 13.096, n = 4; 1 mm 8 Br 1.025% ± 15.822, n = 4; and 5 mm 8 Br 8.72% ± 7.435, n = 5.
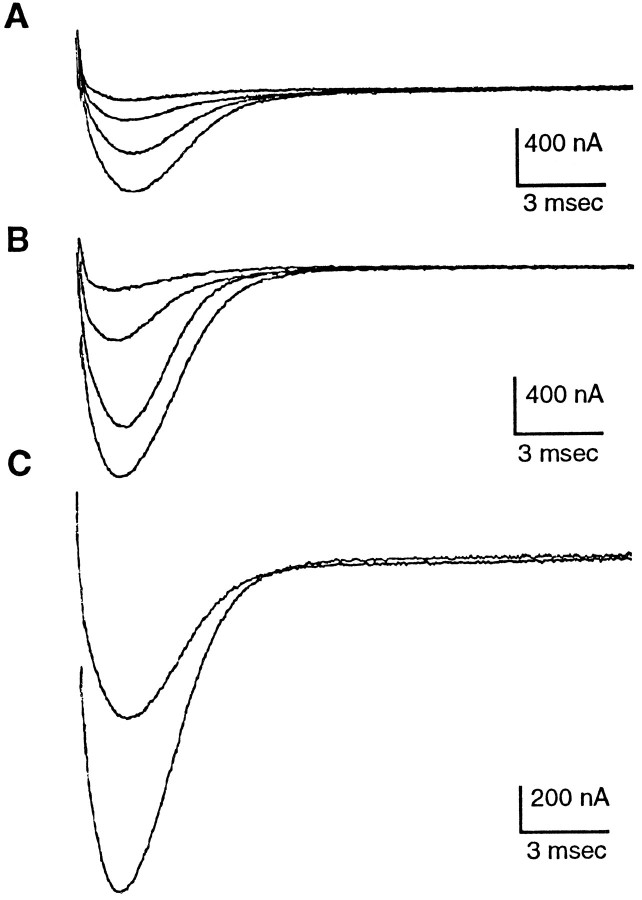
Fig. 1.
Na+ currents at membrane potentials in steps of 10 mV from −15 to +15 mV (peak current in this cell). A, Currents elicited in normal recording saline.B, Currents from the same cell elicited after 20 min exposure to 5 mm 8 Br cAMP. C, Peak currents from A and B superimposed.
Other voltage-dependent characteristics of the Na+ current were not affected by treatment with 8 Br cAMP (Fig. 2). There was a slight shift (∼5 mV) in voltage of peak current in the hyperpolarizing direction, but this was seen at 20 min in the saline control group as well as in the experimental group (Fig. 2A,B). There were no differences between baseline and treatment in steady-state inactivation (Fig. 2C,D) or recovery from inactivation (Fig.2E,F).
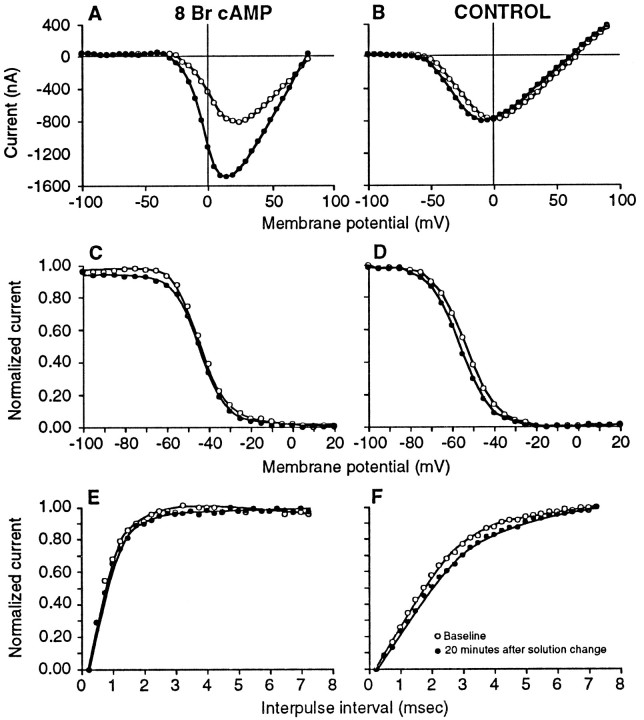
Fig. 2.
Treatment of electrocytes with 8 Br cAMP does not alter voltage-dependent parameters of the Na+current. Open circles, Currents recorded in saline to obtain baseline values; closed circles, currents recorded 20 min after adding 5 mm 8 Br cAMP (A, C, Efrom the same cell in fish No. 553) or fresh saline (Controls B, D, F from the same cell in fish No. 202). Current–voltage plots of representative cells illustrate that although voltage of peak current became slightly more hyperpolarized by 20 min, this was a nonspecific effect seen in control (B) as well as treated (A) cells. In this case, the Na+current magnitude increased almost twofold in response to 8 Br cAMP. No differences between baseline and either 8 Br cAMP (C) or control (D) steady-state inactivation were seen (decrease in magnitude of Na+ current to a standard test pulse at 0 mV caused by 25 msec prepulses at the voltages indicated on the abscissa). Curves fit by Boltzmann equation. Recovery from inactivation before and after treatment with 5 mm 8 Br cAMP (E) or saline (F) did not change from baseline [increase in magnitude of the Na+ current to the second of two standard test pulses (25 msec, 0 mV) as the interval between them is increased]. Curves fit by eye. Differences in the slopes of curves between the cell from fish No. 202 illustrated in theright side of the figure (B, D, F) and that from fish No. 553 on the left (A, C, E) are indicative of the individual variation in these parameters between fish.
Steady-state membrane resistance increased slightly (∼5% at 20 min) over time in all groups (range for all treatment groups 2.378–8.916%).
Time course of PKA activation
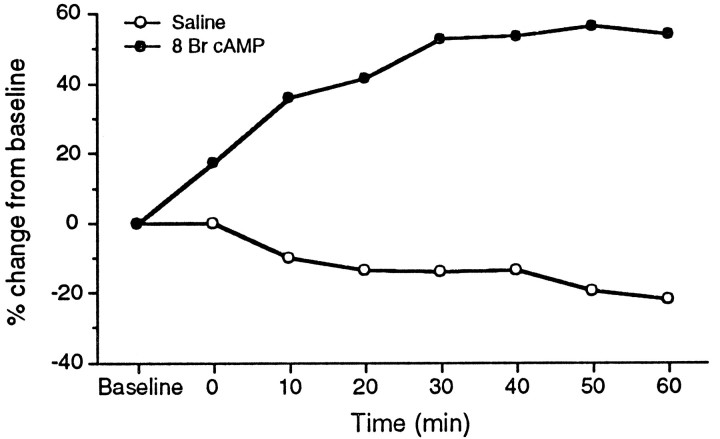
When we were able to hold the cell for sufficient time, current magnitude continued to increase through at least the first 30 min of recording and often was still increasing at 1 hr (Fig.3). In a few cells, current plateaued or decreased slightly after 30 min. However, in no cases did the magnitude return to baseline level. In contrast, in cells in recording saline alone, the Na+ current often declined slightly over time.
Fig. 3.
Time course of PKA activation in two representative cells. Closed circles, 1 mm 8 Br cAMP; open circles, saline control. 8 Br cAMP produced a sustained increase in Na+ current magnitude in contrast to a gradual decay in the current seen in controls.
We were reliably able to maintain a good voltage clamp for at least 20 min. In addition, at least half of the total increase in current produced by 8 Br cAMP occurred by 20 min. Therefore, we used 20 min as a criterion time in further experiments to quantify the effect of 8 Br cAMP.
Dose–response of the effect of 8 Br cAMP
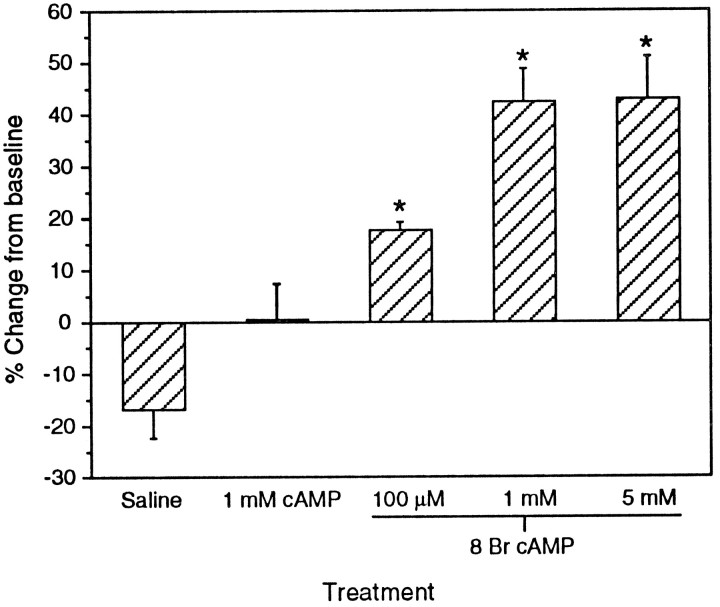
We examined the effect of 20 min exposure to 100 μm, 1 mm, and 5 mm 8 Br cAMP, as well as 1 mm cAMP, on the Na+current. All doses of 8 Br cAMP produced increases in current magnitude that were significantly different from saline control atp < 0.05 (Fig. 4, one-way ANOVA overall, p = 0.0001). The Na+current increase was dose-dependent, with a greater effect on the current magnitude at 1 mm (mean ± SEM = 42.36 ± 6.39%) than at 100 μm (17.64 ± 1.64%). The effect plateaued at 1 mm; there was no difference between 1 and 5 mm (43.06 ± 8.06%). As noted previously, cells left in recording saline showed a decline in Na+ current over time. Cyclic AMP (0.6 ± 6.88%), which does not permeate the cell membrane as readily as 8 Br cAMP, did not increase the current magnitude significantly compared with saline (−16.8 ± 5.41%), although it seemed to prevent the decline observed in saline.
Fig. 4.
Dose–response to 8 Br cAMP. The enhancement of the Na+ current by 8 Br cAMP was significantly different from saline at all doses tested (one-way ANOVA, overallp = 0.0001, treatments different from each other atp ≤ 0.05, Fisher protected least significant difference). Cyclic AMP was not significantly different from saline.n = 5 in all treatments.
Effect of PKA inhibition
To determine whether the effect we observed with cAMP analog treatment could be blocked specifically by PKA inhibition, we pretreated electrocytes in which a voltage clamp had been established (in regular recording saline) with 10 ml of recording saline containing 30 μm H89 for at least 20 min. New baseline recordings were made in this solution. We then replaced the H89 solution with 10 ml of saline containing both 30 μm H89 and 1 mm 8 Br cAMP and examined voltage-clamp parameters as described in the previous experiment. In control experiments, DMSO was added to recording saline containing 1 mm 8 Br cAMP at a final concentration of 1%.
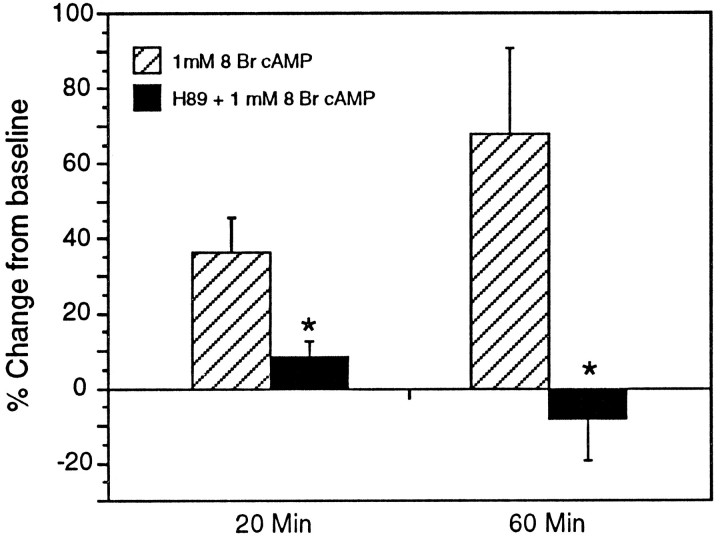
The effect of 8 Br cAMP on Na+ current magnitude was not influenced by DMSO, but was significantly blocked by treatment with H89 (Fig. 5). At 20 min, Na+current magnitude increased by an average of 36.36% (±9.45% SEM) in 8 Br cAMP treated cells, but only by 8.42% (±9.78) in cells also treated with H89. This difference was significant at p = 0.0173 (unpaired two-tailed t test). By 60 min, whereas the Na+ current magnitude continued to increase in 8 Br cAMP-treated cells to 67.8% (±22.88%) above baseline values, it had declined to −8.06% (± 11.27%) below baseline in those treated with H89 (p = 0.0154). Na+ currents in cells treated with H89 alone declined slightly (an average of 11.6% by 20 min). This was similar to cells in saline alone.
Fig. 5.
H89 (30 μm) blocked the effect of 1 mm 8 Br cAMP on Na+ current magnitude. At 20 min, the Na+ current in cells that had been pre- and cotreated with H89 (n = 6) was only 23% of that from cells treated with 8 Br cAMP alone (n = 5). By 60 min, the difference was even more pronounced because current magnitude continued to increase in cells treated with 8 Br cAMP alone (n = 4, one cell was lost before 60 min) but declined relative to baseline values with H89 treatment (n = 5, one cell was lost before 60 min).
τh was not significantly affected by H89; at 20 min, mean percent change from baseline in τhwas −2.367% ± 12.099 for 8 Br cAMP-treated cells (n = 6) and 3.8% ± 10.606 for cells treated with H89 + 8 Br cAMP (n = 5). Other voltage-dependent parameters were not significantly different between the two groups.
DISCUSSION
Technical considerations
We observed two nonspecific changes in the Na+ current: a gradual decrease in current amplitude in cells exposed to saline only, and a slight shift in the hyperpolarizing direction of the voltage of peak current in saline as well as in 8 Br cAMP-treated cells. These changes were not attributable to deterioration of the voltage clamp with a concomitant decrease in membrane resistance, because membrane resistance increased slightly on average in all groups, and we saw no indication that the clamp deteriorated. Nonspecific, time-dependent shifts in voltage-dependent parameters are observed in other systems (Grant and Wendt, 1992; Wendt et al., 1992; Ma et al., 1994).
The rundown that we observed in Na+ current is also similar to that seen in other voltage-clamp studies of the Na+ channel (Herzig and Kohlhardt, 1991; Smith and Goldin, 1992). In eel electrocytes, Shenkel and Sigworth (1991)observed a decrease of ≥95% in Na+ currents recorded in excised patches from tissue that had been refrigerated overnight in normal saline. This effect was not observed in tissue stored in Ca2+-free saline, suggesting that an increase in intracellular Ca2+ may depress Na+ currents.
PKA mediates the cAMP-induced increase in Na+ current
We observed a significant increase in Na+current magnitude with the lowest concentration of 8 Br cAMP used (100 μm) and a saturation of the effect at 1–5 mm. We were able to block the effect of a saturating concentration of 8 Br cAMP with the PKA inhibitor H89, supporting the hypothesis that the 8 Br cAMP-induced Na+ current increase was mediated by the PKA pathway.
The lack of an effect of cAMP and inhibition of the 8 Br cAMP-induced increase in the Na+ current by the PKA inhibitor H89 argue against an extracellular site of action as observed in cardiac myocytes (Sorbera and Morad, 1991), as well as a cyclic nucleotide-gated Na+ channel (for review, seeZimmerman, 1995).
Comparison with the effect of PKA on eel electrocytes and other cells
The most closely related species in which phosphorylation of Na+ channels has been examined is the eel.Emerick et al. (1993) found that exposure of inside-out membrane patches from Sachs organ to PKA resulted in an 80% reduction in Na+ current amplitude and a 10–12 mV negative shift in the current–voltage relation. These results are in apparent contradiction to our own. However, our studies differed in several aspects.
First, we used two-electrode voltage clamp of intact, nondissociated electrocytes in contrast to the eel study, in which excised patches were made from dissociated electrocytes. We did this to minimize any effects of dissociating enzymes on extracellular protein domains and the possible loss of cytoplasmic factors via dialysis and patch removal. In addition, any interactions between the Na+ channel and cytoskeletal elements would be preserved in the intact electrocyte. Such interactions may influence channel function (Adelman, 1995).
Second, in the eel study phosphorylation was induced with an exogenous (bovine cardiac) PKA, whereas we presumably activated native PKA with 8 Br cAMP. Although we did not directly determine that 8 Br cAMP activates PKA, this is likely because the effect of 8 Br cAMP is blocked by the addition of H89.
Third, although Sternopygus is a close relative of the electric eel, the differences in our results may be species differences. A comparison of the effect of PKA activation on Na+ channels from each species under similar recording protocols would be instructive.
In other preparations, PKA-dependent phosphorylation of voltage-dependent Na+ channels is generally reported to decrease current amplitude (Gershon et al., 1992; Grant and Wendt, 1992; Li et al., 1992; Li et al., 1993; Schiffmann et al., 1995). However, current increases have been reported in other studies of similar channels (Smith and Goldin, 1992; Matsuda et al., 1992). The control of channel properties by phosphorylation is complex and depends on many factors. For example, phosphorylation of certain sites can alter the effect of subsequent phosphorylation at other sites. Previous PKC phosphorylation is required for PKA to decrease the current of rat brain IIA Na+ channels expressed in Chinese hamster ovary cells, whereas PKA activation alone either has no affect or increases Na+ currents (Li et al., 1993). We do not yet know the extent to which the SternopygusNa+ channel is endogenously phosphorylated.
Possible mechanisms of the cAMP effect on electrocyte Na+ currents
The most straightforward explanation of our results is that PKA phosphorylates the Na+ channel directly, increasing the probability of channel opening. Nevertheless, PKA could affect Na+ current amplitude by acting on other substrates. For example, PKA could phosphorylate a regulatory subunit associated with the Na+ channel. β subunits, for example, are well known in mammalian tissues, although they do not appear to be phosphorylated by PKA (Hartshorne and Catterall, 1984;Yang et al., 1993). In addition, attempts to identify β subunits in eel or Sternopygus electrocytes have thus far been unsuccessful (Correa et al., 1990; Isom et al., 1992; Lopreato, personal communication).
Rather than acting on channels already in the membrane, cAMP activation could increase current amplitude by inducing synthesis of new Na+ channels as has been observed in other systems (Sherman and Catterall, 1984; Offord and Catterall, 1989; Zhang et al., 1992). However, sodium channels are large, heavily glycosylated proteins; in eel electrocytes, it takes 8–24 hr for new Na+ channels to be transcribed, translated, and post-translationally processed (Thornhill and Levinson, 1987). Because we observed increases in current magnitude within minutes of the addition of 8 Br cAMP, it is unlikely that the mechanism requires transcription of Na+ channel genes.
Another possibility is that PKA phosphorylates a cytoskeletal or vesicular protein that induces the incorporation of mature Na+ channels from a cytoplasmic reservoir. For example, recent reports suggest that vasopressin acts via PKA to insert new amiloride-sensitive Na+ channels into the cell membrane within 5 min to an hour (Marunaka and Eaton, 1991;Kleyman et al., 1994). The time course of the Na+current increase that we observed in Sternopygus is also consistent with this mechanism.
Physiological and behavioral significance
Assuming there is no compensating change in other ion currents, an increase in Na+ current amplitude would result in an increase in AP amplitude and the current generated by the EO. This, in turn, would increase the amplitude of the EOD around the fish.
Behaviorally relevant variation in EOD amplitude occurs in various species of electric fish. EOD amplitude varies diurnally (Franchina, 1993; Hagedorn, 1995) and is increased rapidly in response to physical stimulation or social interactions in some gymnotiforms (Hagedorn and Zelick, 1989; Hagedorn, 1995). EOD amplitude also responds to changes in water conductivity. Because the mormyrid EO acts like a constant current source (Bell et al., 1976), changes in water conductance result in changes in EOD voltage. Kuhn and Kramer (1993) have shown that although EOD amplitude drops after mormyrid fish are placed in highly conductive water, compensatory increases in EOD amplitude (presumably attributable to increased current output of the EO) occur over the next few days. When fish are returned to higher resistance water, EOD amplitude is anomolously high for a few days and then returns to baseline. One mechanism for increasing the current output of the EO would be an increase in Na+ current.
A more detailed understanding of the endocrinological changes associated with changes in EOD amplitude might point to a hormone responsible for initiating the cAMP cascade. Considering the EOD response to changes in water conductivity, for example, changes in prolactin (an osmoregulatory hormone in fish) levels during altered osmoregulatory loads could affect PKA activation, upregulate Na+ currents, and thereby increase current output of the EO. Changes in EO amplitude that occur during courtship (Hagedorn and Heiligenberg, 1985) might be a result of hormones, such as the peptide arginine vasotocin, which is known to be increased during courtship in lower vertebrates (Moore, 1992).
Although we have previously shown that androgens modulate Na+ current kinetics in Sternopygus(Ferrari et al., 1995), it is unlikely that this is mediated by the PKA-dependent changes that we observed in this study. Changes in AP duration were observed only after the fish had been exposed to DHT for a week (Mills and Zakon, 1987), whereas we observed increased Na+ current amplitude within minutes of exposure to 8 Br cAMP. In addition, we observed no effect of 8 Br cAMP on Na+ current kinetics. However, if there are multiple phosphorylation sites in the SternopygusNa+ channel that are differentially phosphorylated by different kinases such as PKC, it is still possible that phosphorylation modulates current kinetics. We plan to examine the effect of PKC activators on the SternopygusNa+ channel in the future. Because, as noted above, the level of endogenous phosphorylation can affect the response obtained by activation of protein kinases, we plan to determine the level of endogenous phosphorylation of electrocyte Na+ channels and to examine the effect of protein phosphatases on these channels.
Footnotes
This work was funded by National Institutes of Health Grant RO1 NS25513 (H.Z.) and Office of Naval Research Grant NOOD14-91-J-1178 (H.Z.)
Correspondence should be addressed to Dr. Lynne McAnelly, Department of Zoology, The University of Texas at Austin, Austin, TX 78712.
REFERENCES
- 1.Adelman JP. Proteins that interact with the pore-forming subunits of voltage-gated ion channels. Curr Opin Neurobiol. 1995;5:286–295. doi: 10.1016/0959-4388(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 2.Bell CC, Bradbury J, Russell CJ (1976) The electric organ of a mormyrid as a current and voltage source. J Comp Physiol 110: 65–88.
- 3.Bennett MVL. Modes of operation of electric organs. Ann NY Acad Sci. 1961;54:458–494. [Google Scholar]
- 4.Bennett MVL. Electric organs. In: Hoar WS, Randall DJ, editors. Fish physiology. Academic; New York: 1971. pp. 347–492. [Google Scholar]
- 5.Bullock TH, Heiligenberg W. Wiley; New York: 1986. Electroreception. . [Google Scholar]
- 6.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Tsutomu I, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N -[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC 12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 7.Correa AM, Bezanilla F, Agnew WS. Voltage activation of purified eel sodium channels reconstituted into artificial liposomes. Biochemistry. 1990;29:6230–6240. doi: 10.1021/bi00478a017. [DOI] [PubMed] [Google Scholar]
- 8.Elliott AA, Elliott JR. Characterization of TTX-sensitive and TTX-resistant sodium currents in small cells from adult rat dorsal root ganglia. J Physiol (Lond) 1993;463:39–56. doi: 10.1113/jphysiol.1993.sp019583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emerick MC, Agnew WS. Identification of phosphorylation sites for adenosine 3′,5′-cyclic phosphate dependent protein kinase on the voltage-sensitive sodium channel from Electrophorus electricus . Biochemistry. 1989;28:8367–8380. doi: 10.1021/bi00447a016. [DOI] [PubMed] [Google Scholar]
- 10.Emerick MC, Shenkel S, Agnew WS. Regulation of the eel electroplax Na channel and phosphorylation of residues on amino- and carboxyl-terminal domains by cAMP-dependent protein kinase. Biochemistry. 1993;32:9435–9444. doi: 10.1021/bi00087a023. [DOI] [PubMed] [Google Scholar]
- 11.Ferrari MB, Zakon HH. Conductances contributing to the action potential of Sternopygus electrocytes. J Comp Physiol [A] 1993;173:281–292. doi: 10.1007/BF00212692. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari MB, McAnelly ML, Zakon HH. Individual variation in and androgen-modulation of the sodium current in electric organ. J Neurosci. 1995;15:4023–4032. doi: 10.1523/JNEUROSCI.15-05-04023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franchina CR. The waveform of the weakly electric fish Hypopomus pinnicaudatus changes daily in the male. J Comp Physiol [A] 1993;173:742. [Google Scholar]
- 14.Geilen CG, Wieprecht M, Wieder T, Reutter W. A selective inhibitor of cyclic AMP-dependent protein kinase, N -[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), inhibits phosphatidylcholine biosynthesis in HeLa cells. FEBS Lett. 1992;309:381–384. doi: 10.1016/0014-5793(92)80811-t. [DOI] [PubMed] [Google Scholar]
- 15.Ginty DD, Glowacka D, Bader DS, Hidaka H, Wagner J A. Induction of immediate early genes by Ca2+ requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991;266:17454–17458. [PubMed] [Google Scholar]
- 16.Gershon E, Weigl L, Lotan I, Schreibmayer W, Dascal N. Protein kinase A reduces voltage-dependent Na+ current in Xenopus oocytes. J Neurosci. 1992;12:3743–3752. doi: 10.1523/JNEUROSCI.12-10-03743.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant AO, Wendt DJ. Block and modulation of cardiac Na+ channels by antiarrhythmic drugs, neurotransmitters and hormones. Trends Pharmacol Sci. 1992;13:352–358. doi: 10.1016/0165-6147(92)90108-i. [DOI] [PubMed] [Google Scholar]
- 18.Hagedorn M. The electric fish Hypopomus occidentalis can rapidly modulate the amplitude and duration of its electric organ discharges. Anim Behav. 1995;49:1409–1413. [Google Scholar]
- 19.Hagedorn M, Heiligenberg W. Court and spark: electric signals in the courtship and mating of gymnotoid electric fish. Anim Behav. 1985;33:254–265. [Google Scholar]
- 20.Hagedorn M, Zelick R. Relative dominance among males is expressed in the electric organ discharge characteristics of a weakly electric fish. Anim Behav. 1989;38:520–525. [Google Scholar]
- 21.Hartshorne RP, Catterall WA. The sodium channel from rat brain: purification and subunit composition. J Biol Chem. 1984;259:1667–1675. [PubMed] [Google Scholar]
- 22.Herzig J, Kohlhardt M. Na+channel blockade by cyclic AMP and other 6-aminopurines in neonatal rat heart. J Membr Biology. 1991;119:163–170. doi: 10.1007/BF01871415. [DOI] [PubMed] [Google Scholar]
- 23.Hille B. Sinauer; Sunderland, MA: 1992. Ionic channels of excitable membranes. 2nd Ed. [Google Scholar]
- 24.Hopkins CD. Sex differences in electric signaling in an electric fish. Science. 1972;176:1035–1037. doi: 10.1126/science.176.4038.1035. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins CD. Electric communication in the reproductive behavior of Sternopygus macrurus (Gymnotidae). Z Tierpsychol. 1974;35:518–535. doi: 10.1111/j.1439-0310.1974.tb00465.x. [DOI] [PubMed] [Google Scholar]
- 26.Isom LL, De Jongh KS, Patton DE, Reber BFX, Offord J, Charbonneau H, Walsh K, Goldin AL, Catterall WA. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science. 1992;256:839–842. doi: 10.1126/science.1375395. [DOI] [PubMed] [Google Scholar]
- 27.Kallen RG, Shen ZH, Yang J, Chen L, Rogart RB, Barchi RL. Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron. 1990;4:233–242. doi: 10.1016/0896-6273(90)90098-z. [DOI] [PubMed] [Google Scholar]
- 28.Kleyman T, Ernst S, Coupaye-Gerard B. Arginine vasopressin and forskolin regulate apical cell surface expression of epithelial Na+ channels in A6 cells. Am J Physiol. 1994;266:F506–F511. doi: 10.1152/ajprenal.1994.266.3.F506. [DOI] [PubMed] [Google Scholar]
- 29.Kuhn B, Kramer B. Constancy of communication and orientation signal of weakly electric fishes (Mormyridae) during changing water conductivity. J Comp Physiol [A] 1993;173:740. [Google Scholar]
- 30.Li M, West J, Lai Y, Scheuer T, Catterall W. Functional modulation of brain sodium channels by cAMP-dependent phosphorylation. Neuron. 1992;8:1151–1159. doi: 10.1016/0896-6273(92)90135-z. [DOI] [PubMed] [Google Scholar]
- 31.Li M, West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. Convergent regulation of sodium channels by protein kinase C and cAMP-dependent protein kinase. Science. 1993;261:1439–1442. doi: 10.1126/science.8396273. [DOI] [PubMed] [Google Scholar]
- 32.Ma J, Catterall W, Scheuer T. Modulation of brain Na+ channels by a G-protein-coupled pathway. Proc Natl Acad Sci USA. 1994;91:12351–12355. doi: 10.1073/pnas.91.25.12351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marunaka Y, Eaton DC. Effects of vasopressin and cAMP on single amiloride-blockable Na channels. Am J Physiol. 1991;260:C1071–C1084. doi: 10.1152/ajpcell.1991.260.5.C1071. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda J, Lee H, Shibata E. Enhancement of rabbit cardiac sodium channels by beta-adrenergic stimulation. Circ Res. 1992;70:199–207. doi: 10.1161/01.res.70.1.199. [DOI] [PubMed] [Google Scholar]
- 35.Meyer JH. Steroid influeces upon the discharge frequency of a weakly electric fish. J Comp Physiol [A] 1983;153:29–38. [Google Scholar]
- 36.Mills AC, Zakon HH. Coordination of EOD frequency and pulse duration in a weakly electric wave fish: the influence of androgens. J Comp Physiol [A] 1987;161:417–430. [Google Scholar]
- 37.Mills AC, Zakon HH. Chronic androgen treatment increases action potential duration in the electric organ of Sternopygus . J Neurosci. 1991;11:2349–2361. doi: 10.1523/JNEUROSCI.11-08-02349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moore FL. Evolutionary precedents for behavioral actions of oxytocin and vasopressin. Ann NY Acad Sci. 1992;652:156–165. doi: 10.1111/j.1749-6632.1992.tb34352.x. [DOI] [PubMed] [Google Scholar]
- 39.Numann R, Catterall WA, Scheuer T. Functional modulation of brain sodium channels by protein kinase C phosphorylation. Science. 1991;254:115–118. doi: 10.1126/science.1656525. [DOI] [PubMed] [Google Scholar]
- 40.Offord J, Catterall W. Electrical activity, cAMP, and cytosolic calcium regulate mRNA encoding sodium channel A subunits in rat muscle cells. Neuron. 1989;2:1447–1452. doi: 10.1016/0896-6273(89)90190-6. [DOI] [PubMed] [Google Scholar]
- 41.Ogata N, Tatebayashi H. Kinetic analysis of two types of Na+ channels in rat dorsal root ganglia. J Physiol (Lond) 1993;466:9–37. [PMC free article] [PubMed] [Google Scholar]
- 42.Rizzo MA, Kocsis JD, Waxman SG. Slow sodium conductances of dorsal root ganglion neurons: intraneuronal homogeneity and interneuronal heterogeneity. J Neurophysiol. 1994;72:2796–2815. doi: 10.1152/jn.1994.72.6.2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schiffmann S, Lledo P, Vincent J. Dopamine D1 receptor modulates the voltage-gated sodium current in rat striatal neurones through a protein kinase A. J Physiol (Lond) 1995;483:95–107. doi: 10.1113/jphysiol.1995.sp020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shenkel S, Sigworth SF. Patch recordings from the electrocytes of Electrophorus electricus . Na current and PNa/Pk variability. J Gen Physiol. 1991;97:1013–1041. doi: 10.1085/jgp.97.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sherman S, Catterall W. Electrical activity and cytosolic calcium regulate levels of tetrodotoxin-sensitive sodium channels in cultured rat muscle cells. Proc Natl Acad Sci USA. 1984;81:262–266. doi: 10.1073/pnas.81.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith RD, Goldin AL. Protein kinase A phosphorylation enhances sodium channel currents in Xenopus oocytes. Am J Physiol. 1992;263:C660–C666. doi: 10.1152/ajpcell.1992.263.3.C660. [DOI] [PubMed] [Google Scholar]
- 47.Sorbera L, Morad M. Modulation of cardiac sodium channels by cAMP receptors on the myocyte surface. Science. 1991;253:1286–1289. doi: 10.1126/science.1653970. [DOI] [PubMed] [Google Scholar]
- 48.Takuma T, Ichida T. Evidence for the involvement of protein phosphorylation in cyclic AMP-mediated amylase exocytosis from parotid acinar cells. FEBS Lett. 1994;340:29–33. doi: 10.1016/0014-5793(94)80167-3. [DOI] [PubMed] [Google Scholar]
- 49.Thornhill W, Levinson S. Biosynthesis of electroplax sodium channels in Electrophorus electrocytes and Xenopus oocytes. Biochemistry. 1987;26:4381–4388. doi: 10.1021/bi00388a029. [DOI] [PubMed] [Google Scholar]
- 50.Wendt DJ, Starmer CF, Grant AO. Na channel kinetics remain stable during perforated-patch recordings. Am J Physiol. 1992;263:C1234–C1240. doi: 10.1152/ajpcell.1992.263.6.C1234. [DOI] [PubMed] [Google Scholar]
- 51.Yang J, Bennett P, Makita N, George A, Barchi R. Expression of the sodium channel β1 subunit in rat skeletal muscle is selectively associated with the tetrodotoxin-sensitive α subunit isoform. Neuron. 1993;11:915–922. doi: 10.1016/0896-6273(93)90121-7. [DOI] [PubMed] [Google Scholar]
- 52.Zakon HH, Thomas P, Yan HY. Electric organ discharge frequency and plasma sex steroid levels during gonadal recrudescence in a natural population of the weakly electric fish Sternopygus macrurus . J Comp Physiol [A] 1991;169:493–499. doi: 10.1007/BF00197661. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J, Robinson R, Siegelbaum S. Sympathetic neurons mediate developmental change in cardiac sodium channel gating through long-term neurotransmitter action. Neuron. 1992;9:97–103. doi: 10.1016/0896-6273(92)90224-2. [DOI] [PubMed] [Google Scholar]
- 54.Zimmerman A. Cyclic nucleotide gated channels. Curr Opin Neurobiol. 1995;5:296–303. doi: 10.1016/0959-4388(95)80041-7. [DOI] [PubMed] [Google Scholar]